- Diseases
- Acoustic Neuroma (14)
- Adrenal Gland Tumor (24)
- Anal Cancer (66)
- Anemia (2)
- Appendix Cancer (16)
- Bile Duct Cancer (26)
- Bladder Cancer (68)
- Brain Metastases (28)
- Brain Tumor (230)
- Breast Cancer (718)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (154)
- Colon Cancer (164)
- Colorectal Cancer (110)
- Endocrine Tumor (4)
- Esophageal Cancer (42)
- Eye Cancer (36)
- Fallopian Tube Cancer (6)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (6)
- Kidney Cancer (124)
- Leukemia (344)
- Liver Cancer (50)
- Lung Cancer (288)
- Lymphoma (284)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (98)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (4)
- Neuroendocrine Tumors (16)
- Oral Cancer (100)
- Ovarian Cancer (170)
- Pancreatic Cancer (164)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (144)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (236)
- Skin Cancer (296)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (60)
- Testicular Cancer (28)
- Throat Cancer (90)
- Thymoma (6)
- Thyroid Cancer (98)
- Tonsil Cancer (30)
- Uterine Cancer (78)
- Vaginal Cancer (14)
- Vulvar Cancer (18)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (20)
- Advance Care Planning (10)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (362)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (628)
- Complementary Integrative Medicine (24)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (230)
- Epigenetics (6)
- Fertility (64)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (124)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (118)
- Molecular Diagnostics (8)
- Pain Management (62)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (898)
- Research (392)
- Second Opinion (74)
- Sexuality (16)
- Side Effects (604)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (404)
- Survivorship (322)
- Symptoms (184)
- Treatment (1776)
Targeting the KRAS mutation for more effective cancer treatment
4 minute read | Published February 12, 2021
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on February 12, 2021
Since the discovery of KRAS gene mutations in 1983, researchers have worked to develop new therapies that target this protein when treating cancer. Unfortunately, KRAS mutations have long been considered impossible to treat with drugs, but MD Anderson researchers have made recent breakthroughs in developing targeted therapies with promising results.
Shubham Pant, M.D., associate professor of Investigational Cancer Therapeutics and Gastrointestinal Medical Oncology, explains the role of KRAS gene mutations in cancer and what progress is being made to develop KRAS inhibitors.
What is KRAS? How do mutations in KRAS lead to cancer?
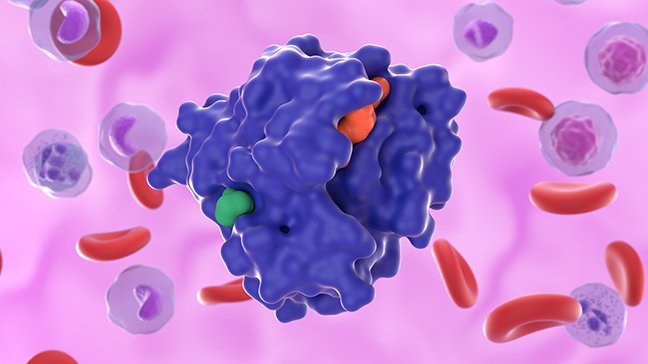
In healthy cells, KRAS serves as an on-off switch that regulates cell growth. It does this by binding a KRAS-activating molecule called GTP and then converts it to GDP, which inactivates the protein.
However, when the gene is mutated, KRAS can become stuck in the “on” position, allowing cells to grow uncontrollably and activating the downstream pathways. This leads to multiplication of cells and cancer growth, which can then cause metastases.
How common are cancers associated with the KRAS mutation? Which cancers are they linked to?
KRAS mutations are present in approximately 25% of tumors, making them one of the most common gene mutations linked to cancer. They are frequent drivers in lung, colorectal and pancreatic cancers. KRAS drives 32% of lung cancers, 40% of colorectal cancers, and 85% to 90% of pancreatic cancer cases. G12C, G12D and G12R are some of the most common KRAS mutations, based on the specific mutations that are present.
How do you determine if someone has the KRAS mutation?
We can determine if someone has the KRAS mutation by conducting genetic sequencing of the tumor tissue or with the help of a liquid biopsy. I would encourage patients to speak with their oncologist about clinical trials specifically targeting this mutation.
Why has KRAS historically been impossible to target with drug treatments?
The KRAS protein is small in size and has a smooth surface – akin to a shiny, smooth ball. This means the protein structure really doesn’t have any deep pockets that drugs can bind to, making it “undruggable.” In addition, it also binds very tightly to GTP in its activated state, which makes blocking activated KRAS challenging.
We haven’t been able to target KRAS mutations with drugs in the past, but newer therapies are now enabling us to directly block specific mutations, like G12C. Through clinical trials, researchers are exploring whether it’s possible to block the downstream signaling pathways, known as the MAPK pathways, which KRAS activates.
What research is being done at MD Anderson to specifically target KRAS mutations?
KRAS is being divided into subsets, such as KRAS G12C, G12D and G12R. It’s like a puzzle: You can potentially divide all these mutations into individual cancers, which are targeted with different agents.
At MD Anderson, we have a number of clinical trials targeting KRAS. One trial is aimed at decreasing the activation of KRAS using son of sevenless (SOS) protein inhibitors. This basically stops the phosphorylation of KRAS and prevents it from entering the activated GTP state. The combination of targeted therapies against SOS and another protein, MEK, has shown promising results in preclinical models. We are now testing that in people with KRAS mutations. It’s really exciting as this is another piece of the puzzle.
We are also launching a study, which is funded by our Pancreatic Cancer Moon Shot®, that inhibits KRAS G12D by combining siRNA with an exosome, called “iExosomes.” These particles are normally used in cell-to-cell communication. However, we believe that if we put the siRNA in it, we may be able to selectively and directly target these KRAS mutations.
Additionally, we have a clinical trial right now that combines MEK inhibitors and hydroxychloroquine to block KRAS in patients with advanced pancreatic cancer. MEK or ERK inhibitors as single agents don’t seem to work in a majority of patients with KRAS mutations. However, we’ve seen in pancreatic cancer models that blocking the MEK-ERK pathway can increase autophagy in these cancer models. Using hydroxychloroquine to block autophagy, we see responses in tumor models, such as pancreatic cancer.
What excites you most about the future of KRAS inhibitors?
With the advent of newer targeted therapies in the past decade, we now have options for treating KRAS mutations with drugs. By combining direct inhibitors and downstream inhibitors of KRAS, the field is entering an exciting era of innovative clinical trials that have the potential to improve outcomes and improve survival for patients with this mutation.
Refer a patient to MD Anderson online or by calling 1-855-634-0528.

With the advent of newer targeted therapies in the past decade, we now have options for treating KRAS mutations with drugs.
Shubham Pant, M.D.
Physician & Researcher