- Diseases
- Acoustic Neuroma (14)
- Adrenal Gland Tumor (24)
- Anal Cancer (68)
- Anemia (2)
- Appendix Cancer (16)
- Bile Duct Cancer (26)
- Bladder Cancer (72)
- Brain Metastases (28)
- Brain Tumor (232)
- Breast Cancer (718)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (162)
- Colon Cancer (166)
- Colorectal Cancer (118)
- Endocrine Tumor (4)
- Esophageal Cancer (44)
- Eye Cancer (36)
- Fallopian Tube Cancer (8)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (14)
- Kidney Cancer (128)
- Leukemia (342)
- Liver Cancer (50)
- Lung Cancer (286)
- Lymphoma (278)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (100)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (6)
- Neuroendocrine Tumors (16)
- Oral Cancer (100)
- Ovarian Cancer (174)
- Pancreatic Cancer (160)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (148)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (238)
- Skin Cancer (296)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (64)
- Testicular Cancer (28)
- Throat Cancer (92)
- Thymoma (6)
- Thyroid Cancer (98)
- Tonsil Cancer (30)
- Uterine Cancer (84)
- Vaginal Cancer (18)
- Vulvar Cancer (22)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (20)
- Advance Care Planning (12)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (362)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (632)
- Complementary Integrative Medicine (22)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (232)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (126)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (116)
- Molecular Diagnostics (8)
- Pain Management (62)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (924)
- Research (392)
- Second Opinion (76)
- Sexuality (16)
- Side Effects (610)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (402)
- Survivorship (322)
- Symptoms (182)
- Treatment (1788)
Papillary thyroid cancer survivor: Targeted therapy is giving me a future
3 minute read | Published March 04, 2020
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on March 04, 2020
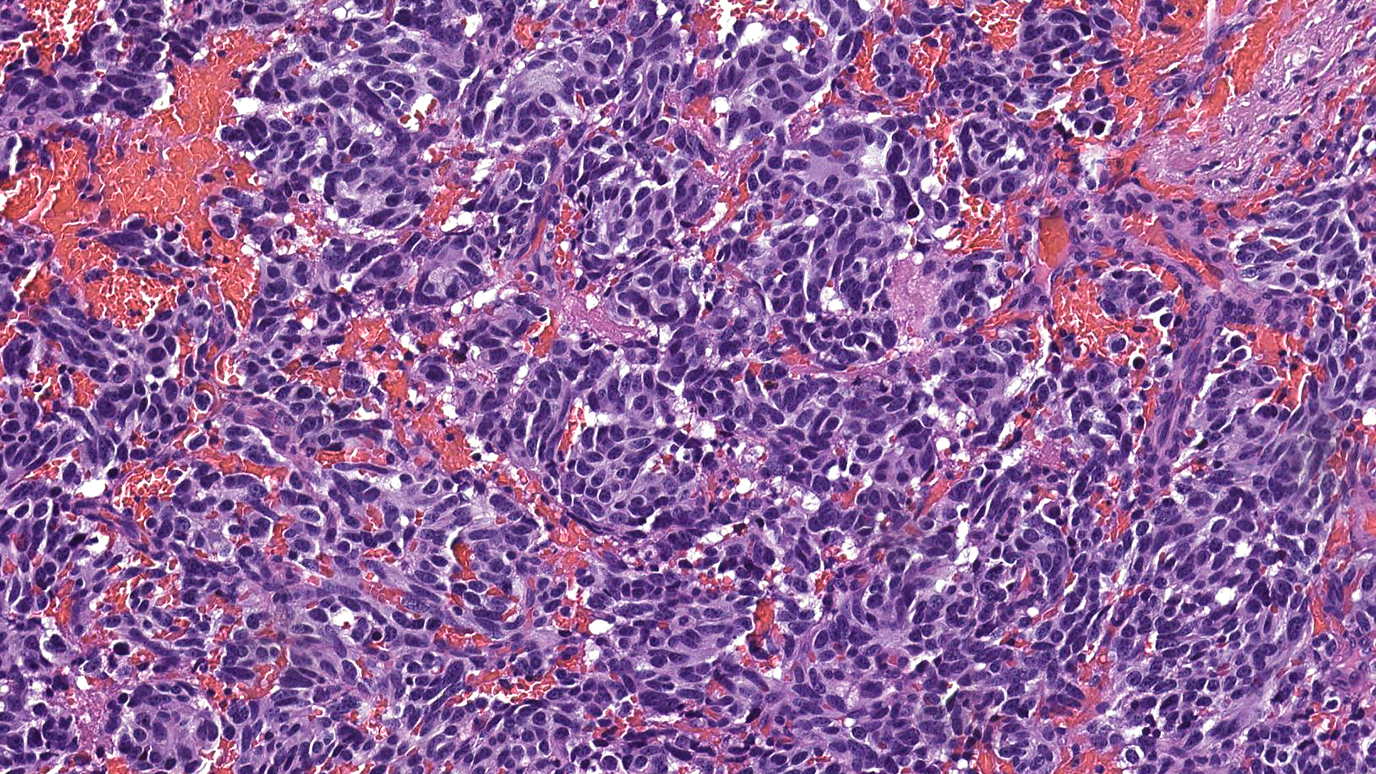
I’ve been living with papillary thyroid cancer since 2012. If it weren’t for MD Anderson, I might not be able to say that. Because when I was first diagnosed, doctors near my home in Dallas suggested hospice.
I was only 17 at the time and had just started treatment, so I wasn’t ready to give up. That’s why I went to MD Anderson. And not only has it given me the past eight years of my life back; it’s also given me a future.
Choosing MD Anderson for my thyroid cancer treatment
My initial appointment at MD Anderson was in April 2013. There, I met with endocrine specialist Dr. Anita Ying. The first thing she recommended was a radioactive iodine pill to kill any residual thyroid tissue I might have. Doctors in Dallas had surgically removed that gland as a part of my treatment there, but some stray cells could still have been left behind. After that, I took two targeted therapy drugs called lenvatinib and sorafenib.
All of those treatments had at least some positive effect. But by the time I was diagnosed, the thyroid cancer had already spread to my lungs. Even with treatment, it never really went away. That’s why Dr. Ying recommended a Phase I/II clinical trial under Dr. Vivek Subbiah. He was testing a new targeted therapy drug called pralsetinib, which has shown very promising results in patients with thyroid and lung cancers.
Why I joined a clinical trial
I’ve always been kind of interested in medicine, so I understood what was being asked of me when I was told I qualified to join the clinical trial. I wasn’t really scared, just a little nervous. But I think it’s amazing to be able to help other people by participating, so I jumped at the offer.
I figured there was a 50/50 chance it would work, so why not? It’s my life. And I feel like if you have a chance to make things better, you should go for it.
How the clinical trial affected me
I joined the clinical trial in March 2018. And after receiving my first dose of the targeted therapy drug, I started feeling better right away. My energy levels improved, too. The only side effect I’ve had so far from any of my treatments is high blood pressure. I take another medication to control it. But as someone who was born with spina bifida, I’ve had to take medications all my life, so that doesn’t bother me.
I still have some small spots of cancer on my lungs, but I no longer need an oxygen mask to help me breathe, and much of the disease has disappeared. The little that remains is pretty stable, so unless the pralsetinib stops working, I’ll keep taking it daily.
Why I’m grateful to MD Anderson
Today, I am super grateful to MD Anderson — and not just for stabilizing my cancer. The $1,200 scholarship I received through its Adolescent and Young Adult (AYA) Program in 2019 is also helping me realize my college dreams.
Having MD Anderson’s support in all of these different areas means so much. It shows how much its leaders care and want what’s best for me and other patients — not only when it comes to our health, but also in regard to our future.
Request an appointment at MD Anderson online or by calling 1-877-632-6789.

If you have a chance to make things better, you should go for it.
Morgan Romero
Survivor