- Diseases
- Acoustic Neuroma (16)
- Adrenal Gland Tumor (24)
- Anal Cancer (70)
- Anemia (2)
- Appendix Cancer (18)
- Bile Duct Cancer (26)
- Bladder Cancer (74)
- Brain Metastases (28)
- Brain Tumor (234)
- Breast Cancer (726)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (164)
- Colon Cancer (168)
- Colorectal Cancer (118)
- Endocrine Tumor (4)
- Esophageal Cancer (44)
- Eye Cancer (36)
- Fallopian Tube Cancer (8)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (14)
- Kidney Cancer (130)
- Leukemia (342)
- Liver Cancer (50)
- Lung Cancer (286)
- Lymphoma (278)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (100)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (6)
- Neuroendocrine Tumors (16)
- Oral Cancer (102)
- Ovarian Cancer (178)
- Pancreatic Cancer (160)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (150)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (238)
- Skin Cancer (300)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (66)
- Testicular Cancer (28)
- Throat Cancer (92)
- Thymoma (6)
- Thyroid Cancer (100)
- Tonsil Cancer (30)
- Uterine Cancer (86)
- Vaginal Cancer (18)
- Vulvar Cancer (22)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (22)
- Advance Care Planning (12)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (360)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (628)
- Complementary Integrative Medicine (22)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (238)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (128)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (122)
- Molecular Diagnostics (8)
- Pain Management (62)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (936)
- Research (390)
- Second Opinion (78)
- Sexuality (16)
- Side Effects (616)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (408)
- Survivorship (328)
- Symptoms (182)
- Treatment (1788)
6 things we’ve learned about CAR T cell therapy
7 minute read | Published November 01, 2022
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on November 01, 2022
Since MD Anderson’s first CAR T cell therapy clinical trial launched in 2015, we’ve cared for hundreds of patients undergoing CAR T cell therapy. Over the years, we’ve gained valuable insight into this game-changing treatment that trains a patient’s immune system to find and kill cancer cells.
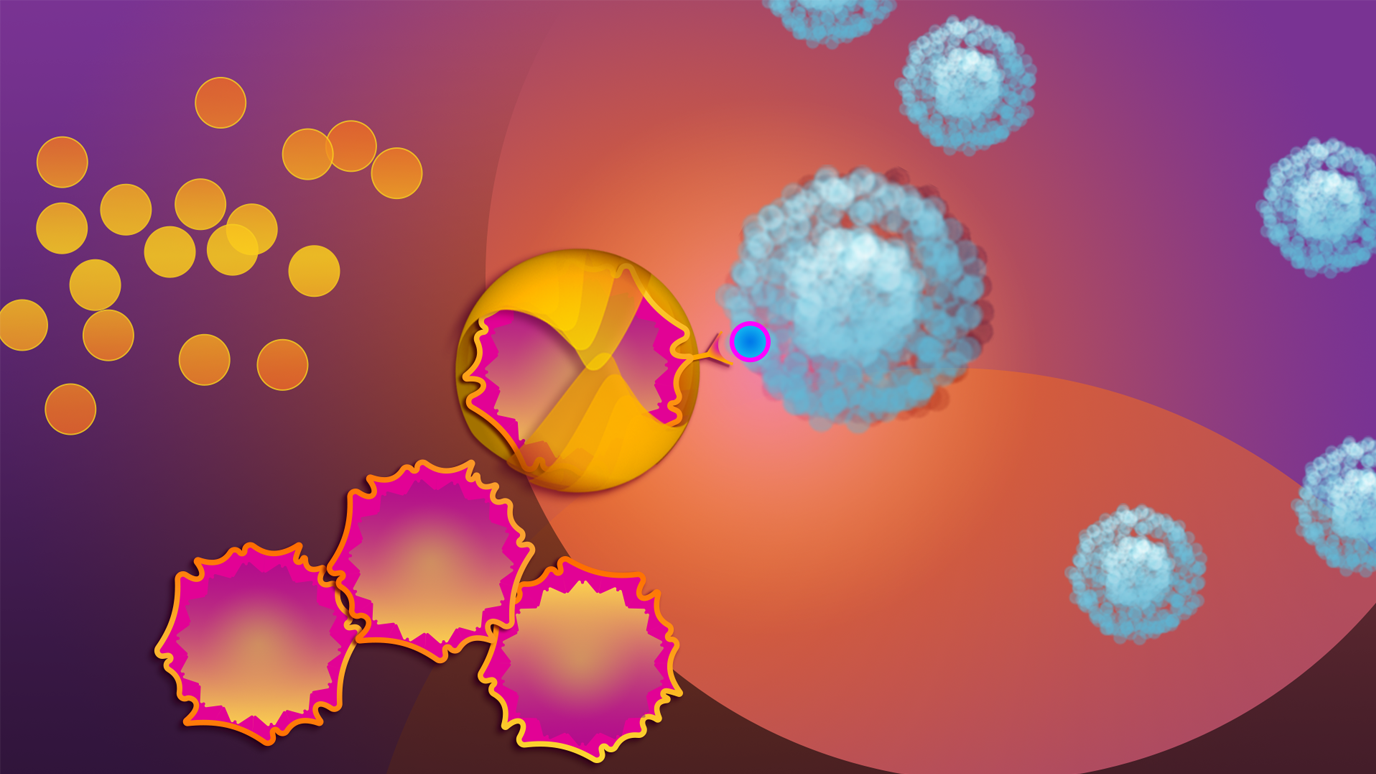
With CAR T cell therapy, T cells that defend the body against disease are extracted from a cancer patient’s blood. The cells are then changed in the lab by adding a protein called chimeric antigen receptor, or CAR. This protein acts like a navigation system that helps the T cells track down and kill cancer cells. These altered T cells – now called CAR T cells – are then multiplied in the lab and reinfused into the patient’s bloodstream.
Today, CAR T cell therapy is approved by the Food and Drug Administration (FDA) to treat certain types of leukemia, lymphoma, and most recently, myeloma. The treatment has produced dramatic results for many patients. In some cases, it has eliminated all evidence of cancer.
With each patient we’ve treated, we’ve learned new lessons that have helped us improve how we care for patients today. Here’s what we’ve learned.
Lesson 1: Side effects can be serious, but managed
Like all cancer treatments, CAR T cell therapy can cause side effects. One of the most frequent and serious side effects is cytokine release syndrome, also called a cytokine storm. This occurs when the immune system responds to the infused CAR T cells more aggressively than it should.
Some patients experience mild, flu-like symptoms such as high fever, fatigue and body aches. Others may have more severe symptoms, including a drop in blood pressure or a lack of oxygen in the tissues that may lead to organ failure.
Cytokine release syndrome was an unexpected side effect in the early days of CAR T cell therapy. But now we know that the drug tocilizumab, originally prescribed for juvenile arthritis, reverses most cases fairly quickly.
Another side effect is ICANS, which stands for “immune effector cell-associated neurotoxicity syndrome.” Patients may become confused and disoriented. They may lose the ability to speak for a short time, which mimics a stroke.
We still don’t know exactly why ICANS occurs, but we’ve learned that most patients recover after several days. Steroids, we’ve discovered, are the most effective treatment for ICANS.
Sherry Adkins, an advanced practice nurse who manages CAR T cell clinical trials in MD Anderson’s Lymphoma/Myeloma department, witnessed these side effects firsthand. She realized that the earlier symptoms are treated, the better patients fare. So she collaborated with MD Anderson’s Informatics department to develop a mobile app that uses a grading system to classify the seriousness of a patient’s side effects. The app then quickly conveys to the patient’s doctors and nurses what treatments to administer, based on national guidelines.
The app has become so successful that we’re now sharing it free of charge with cancer centers and other health care professionals throughout the world.
Lesson 2: Earlier CAR T cell therapy produces better results
In the past, CAR T cell therapy was approved only for patients who had undergone two unsuccessful treatments – typically chemotherapy and a stem cell transplant. Their cancer either didn’t respond, or it responded at first, but then worsened. CAR T cell therapy was a last resort when other treatments failed.
But now we’re starting to prescribe CAR T cell therapy for some patients who’ve had only one type of treatment that failed to work. Early data from clinical trials suggest that CAR T cell therapy is better at treating some cancers than other therapies.
One such trial is ZUMA-12, led by our Sattva Neelapu, M.D. The study found that patients with large B-cell lymphoma who received CAR T cell therapy as part of their initial treatment achieved much higher complete remission rates than patients who did not receive CAR T cell therapy at the start of their treatment.
With ongoing clinical trials producing similar results, we predict CAR T cell therapy will become the first prescribed treatment for some cancers very soon.
Lesson 3: CAR T cell therapy can treat more patients than we initially realized
When CAR T cell therapy first debuted, patients could obtain it only by enrolling in a clinical trial. The treatment was experimental, and not yet approved by the FDA.
Clinical trials have strict inclusion and exclusion criteria, meaning people can enroll only if they meet certain conditions. Typically, they must fall within a certain age range, have no or very limited chronic health conditions, not take medications that would interfere with the trial drug, not be pregnant, and have good liver and kidney function – those two organs play a crucial role in how drugs are metabolized in the body.
These criteria excluded many cancer patients whose only hope for survival was CAR T cell therapy.
Our Loretta Nastoupil, M.D., conducted a clinical study that showed patients who don’t meet eligibility criteria for CAR T cell trials do just as well as patients who do meet the criteria. Thanks to her research, patients no longer need to enroll in clinical trials to obtain CAR T cell therapy.
This has made it possible for us to treat a much larger number of patients with lifesaving CAR T cell therapy. In many of these cases, the cancer has spread.
Lesson 4: We must help CAR T cells work harder and last longer
Many patients achieve complete remission with CAR T cell therapy that essentially cures their disease. But for some, the cancer comes back. We don’t know why this happens. Perhaps the CAR T cells become exhausted and stop working. Or maybe they get crowded out by ordinary T cells that weren’t re-engineered with the CAR protein. Or perhaps patients’ other drugs and treatments interfere with CAR T cells.
We’re working now to learn how to make CAR T cells work better and last longer. We’re seeking answers to questions such as: Do multiple CAR T cell infusions work better than just one? Can certain drugs improve, or worsen, CAR T cells’ persistence and potency? Can we add genes to CAR T cells to make them stronger, or remove genes that make them weaker?
CAR T cell “persistence and potency” is a hot topic in the cancer community, and many studies are underway to increase our knowledge.
Lessen 5: CAR T cells’ ability to treat solid tumors is limited but improving
CAR T cell therapy has revolutionized treatment for blood cancers, but its ability to treat solid tumors remains limited for four reasons: The proteins on solid tumors that CAR T cells zero in on are also found at low levels on the surface of normal cells, making it difficult for the CAR T cells to distinguish tumor cells from healthy ones; the proteins targeted by CAR T cells sometimes hide inside tumor cells and escape detection; cancer cells in the blood are easier to access, since CAR T cells are infused directly into the bloodstream, and; CAR T cells became exhausted when trying to penetrate solid tumors.
Researchers are working to overcome these challenges. Progress is being made, especially in lung, breast and brain cancers. Given the large number of ongoing clinical trials, we’ll soon see CAR T cell treatments designed for solid tumors.
Lesson 6: The demand for CAR T cells is outpacing the supply
There are at least 60,000 patients with blood cancers in the United States each year for whom conventional treatments don’t work. To provide CAR T cells to this many people, we need to ramp up manufacturing.
Companies can’t make CAR T cells fast enough. There simply aren’t enough manufacturers to keep up with the demand. Because the therapy is custom-made for each patient using their own cells, it’s a slow process. Typically, it takes five to six weeks before a patient’s CAR T cells are ready to be infused. Some patients can’t wait that long.
So we’re looking at other alternatives, like using T cells from healthy donors to mass-produce an off-the-shelf option. The CAR T cells would be available immediately, waiting in the freezer.
Other cell therapies similar to CAR T cell therapy are also being studied for potential off-the-shelf options, including CAR NK (natural killer) and TCR (T cell receptor) cell therapies.
Katy Rezvani, M.D., Ph.D., and her team are adding CAR to natural killer cells gathered from umbilical cord blood that is donated by parents after a baby’s birth. A single cord blood donation yields hundreds of doses of CAR NK cell therapy to benefit multiple patients. Her cord blood-derived NK cells have been highly successful in clinical trials of patients who achieved remission with chemotherapy and stem cell transplants only to see their cancer return. She’s now exploring CAR NK cells for the treatment of many types of solid-tumor cancers.
CAR T cell therapy can be a lifesaving treatment
It’s been less than a decade since our first patient received CAR T cell therapy. Although there is still work to be done, the treatment has been lifesaving for many. A significant number of patients treated with CAR T cells will be long-term survivors. That’s phenomenal, considering many were given a slim to none chance of survival.
Request an appointment at MD Anderson online or by calling 1-855-588-9826.
Related Cancerwise Stories

With each patient we’ve treated, we’ve learned new lessons that have helped us improve how we care for patients today.
Sairah Ahmed, M.D.
Physician