- Diseases
- Acoustic Neuroma (16)
- Adrenal Gland Tumor (24)
- Anal Cancer (70)
- Anemia (2)
- Appendix Cancer (18)
- Bile Duct Cancer (26)
- Bladder Cancer (74)
- Brain Metastases (28)
- Brain Tumor (234)
- Breast Cancer (728)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (164)
- Colon Cancer (168)
- Colorectal Cancer (118)
- Endocrine Tumor (4)
- Esophageal Cancer (44)
- Eye Cancer (36)
- Fallopian Tube Cancer (8)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (14)
- Kidney Cancer (130)
- Leukemia (342)
- Liver Cancer (50)
- Lung Cancer (286)
- Lymphoma (278)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (100)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (6)
- Neuroendocrine Tumors (16)
- Oral Cancer (102)
- Ovarian Cancer (178)
- Pancreatic Cancer (162)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (150)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (238)
- Skin Cancer (302)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (66)
- Testicular Cancer (28)
- Throat Cancer (92)
- Thymoma (6)
- Thyroid Cancer (100)
- Tonsil Cancer (30)
- Uterine Cancer (86)
- Vaginal Cancer (18)
- Vulvar Cancer (22)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (22)
- Advance Care Planning (12)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (360)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (628)
- Complementary Integrative Medicine (22)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (240)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (128)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (122)
- Molecular Diagnostics (8)
- Pain Management (62)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (940)
- Research (390)
- Second Opinion (78)
- Sexuality (16)
- Side Effects (616)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (408)
- Survivorship (330)
- Symptoms (182)
- Treatment (1794)
Does immunotherapy treat breast cancer?
BY Devon Carter
4 minute read | Published March 26, 2021
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on March 26, 2021
Last updated March 22, 2022.
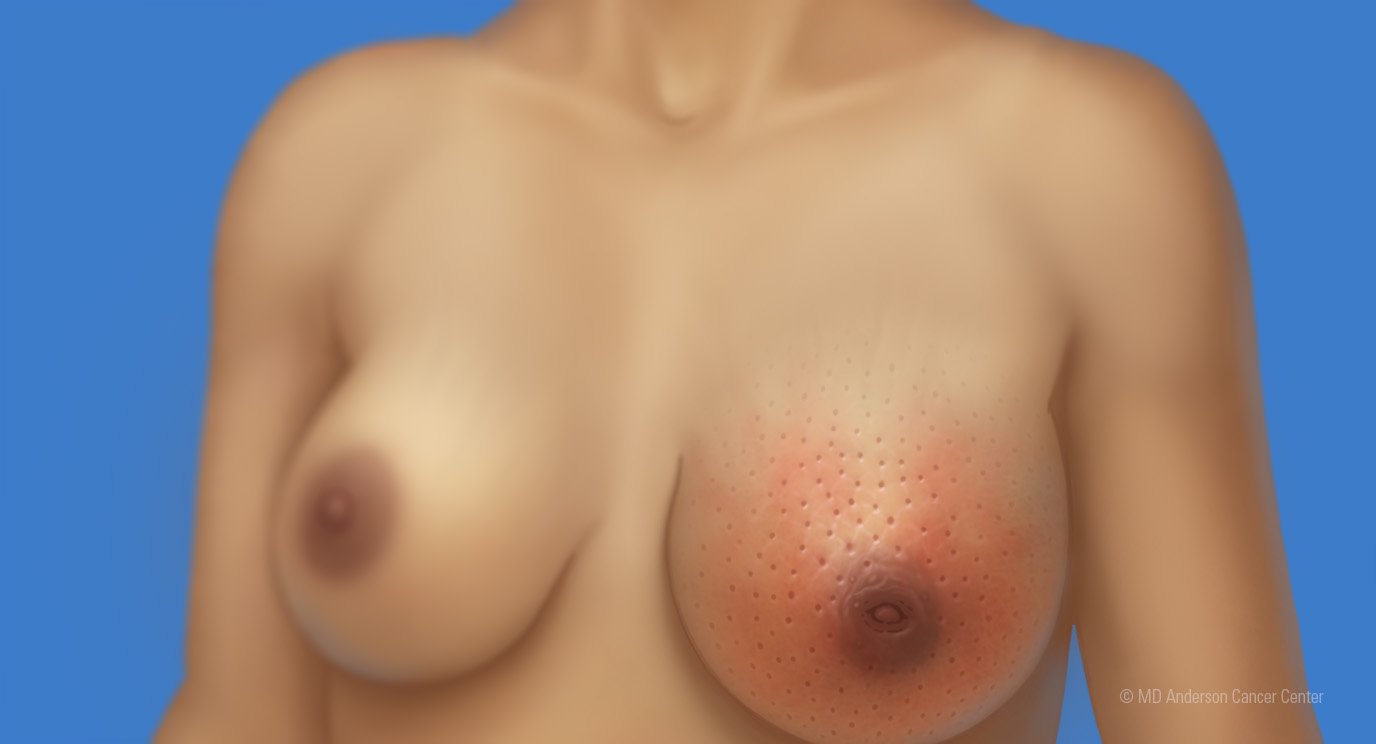
Can immunotherapy treat breast cancer? For certain patients, the answer is yes.
Pembrolizumab is approved by the Food and Drug Administration (FDA) for some patients with metastatic and early-stage triple-negative breast cancer. It’s an immune checkpoint inhibitor, the most common type of immunotherapy.
To understand how the drug works, which breast cancer patients are benefiting and what’s ahead with research, we spoke with Clinton Yam, M.D.
How does immunotherapy work to treat triple-negative breast cancer?
Immunotherapy treats cancers by waking the immune system’s natural ability to defend the body from infection and disease, including cancer. T cells are a type of immune cell that help lead the immune system’s response to an illness.
PD-L1 is a protein that can be found on the surface of cancer cells and/or immune cells. It binds with a partner protein called PD-1 that’s on the surface of a T cell. But this bond turns off the T cell and allows the cancer cell to hide from the T cell. Immune checkpoint inhibitors take off this brake, which increases the likelihood that the T cells will attack and destroy the cancer cells.
Is immunotherapy an option for all patients with triple-negative breast cancer?
Pembrolizumab is currently approved to treat some patients with early-stage or metastatic triple-negative breast cancer.
For patients with metastatic triple-negative breast cancer, immunotherapy in combination with chemotherapy is an option if the PD-L1 protein is detected on the cancer and/or immune cells within the tumor. We determine if a patient has a high expression of PD-L1 through an immunohistochemistry test. This is performed on tissue that’s removed during a biopsy or a previous surgery. If the PD-L1 protein is not found on the cancer cells and/or immune cells within the tumor in patients with metastatic triple-negative breast cancer, a PD-1 immune checkpoint inhibitor won’t be effective.
Patients with high-risk, early-stage disease will be eligible to receive a combination of pembrolizumab and chemotherapy before surgery. After surgery, patients will continue receiving pembrolizumab alone. While “high risk” isn’t defined by the FDA, most providers rely on the definition used in the clinical trial that led to the approval of pembrolizumab. Specifically, this includes patients with breast tumors larger than 2 cm. Patients with smaller breast tumors may be eligible to receive immunotherapy if there is evidence of cancer in the lymph nodes.
Importantly, immunotherapy may worsen symptoms of autoimmune disorders like lupus and rheumatoid arthritis, so these drugs aren’t typically used in women with these chronic illnesses.
What are the side effects of immunotherapy in patients with breast cancer?
Immunotherapy side effects are the same, regardless of the type of cancer being treated.
Immunotherapy increases the ability of the immune system to attack cancer cells, but it also increases the chances that the immune system will affect normal cells. This can cause inflammation throughout the body, which can lead to side effects, such as skin changes, cough, chest pain and diarrhea. In some cases, these side effects can be debilitating, depending on the organ(s) involved.
For example, if the thyroid gland is affected, the patient may develop a condition called hypothyroidism (low thyroid hormone) and may need thyroid hormone replacement in the future. In rare but extreme cases, liver or kidney failure can develop.
When treating breast cancer, is immunotherapy used alone or in combination with other therapies?
Right now, pembrolizumab is used in combination with chemotherapy to treat metastatic triple-negative breast cancer.
However, there are ongoing clinical trials studying other combinations. For example, researchers are trying to find out if immunotherapy is more effective when combined with targeted therapies, such as PARP inhibitors. One challenge with treating breast cancer with immunotherapy is that immune cells have a harder time infiltrating the tumors. By combing immune checkpoint inhibitors with targeted therapies, we hope to help the immune cells better enter the tumor and, therefore, make these drugs an effective treatment option for more patients.
There is also hope that we may be able to use therapeutic vaccines to train the immune system to fight cancer.
Lastly, we know that radiation therapy helps jump-start the immune system. Through clinical trials, researchers are exploring how we might optimize immunotherapy by combining it with radiation therapy.
What are the immunotherapy options for patients with other breast cancer subtypes?
Clinical trials are working to expand the use of immunotherapy to more patients, including those with other stages of triple-negative breast cancer, as well as other breast cancer subtypes.
If you’re interested in immunotherapy options for breast cancer treatment, I encourage you to talk with your care team about clinical trial options. You may find a clinical trial opportunity that can give you access to immunotherapy and also enable you to pay it forward for future cancer patients.
Request an appointment at MD Anderson online or by calling 1-877-632-6789.
Related Cancerwise Stories

You may find a clinical trial opportunity that can give you access to immunotherapy and also enable you to pay it forward for future cancer patients.
Clinton Yam, M.D.
Physician