- Diseases
- Acoustic Neuroma (16)
- Adrenal Gland Tumor (24)
- Anal Cancer (70)
- Anemia (2)
- Appendix Cancer (18)
- Bile Duct Cancer (26)
- Bladder Cancer (74)
- Brain Metastases (28)
- Brain Tumor (234)
- Breast Cancer (728)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (164)
- Colon Cancer (168)
- Colorectal Cancer (118)
- Endocrine Tumor (4)
- Esophageal Cancer (44)
- Eye Cancer (36)
- Fallopian Tube Cancer (8)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (14)
- Kidney Cancer (130)
- Leukemia (342)
- Liver Cancer (50)
- Lung Cancer (286)
- Lymphoma (278)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (100)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (6)
- Neuroendocrine Tumors (16)
- Oral Cancer (102)
- Ovarian Cancer (178)
- Pancreatic Cancer (162)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (150)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (238)
- Skin Cancer (302)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (66)
- Testicular Cancer (28)
- Throat Cancer (92)
- Thymoma (6)
- Thyroid Cancer (100)
- Tonsil Cancer (30)
- Uterine Cancer (86)
- Vaginal Cancer (18)
- Vulvar Cancer (22)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (22)
- Advance Care Planning (12)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (360)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (628)
- Complementary Integrative Medicine (22)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (240)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (128)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (122)
- Molecular Diagnostics (8)
- Pain Management (62)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (940)
- Research (390)
- Second Opinion (78)
- Sexuality (16)
- Side Effects (616)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (408)
- Survivorship (330)
- Symptoms (182)
- Treatment (1794)
CAR T cell therapy for pediatric patients: What parents should know
6 minute read | Published March 12, 2024
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on March 12, 2024
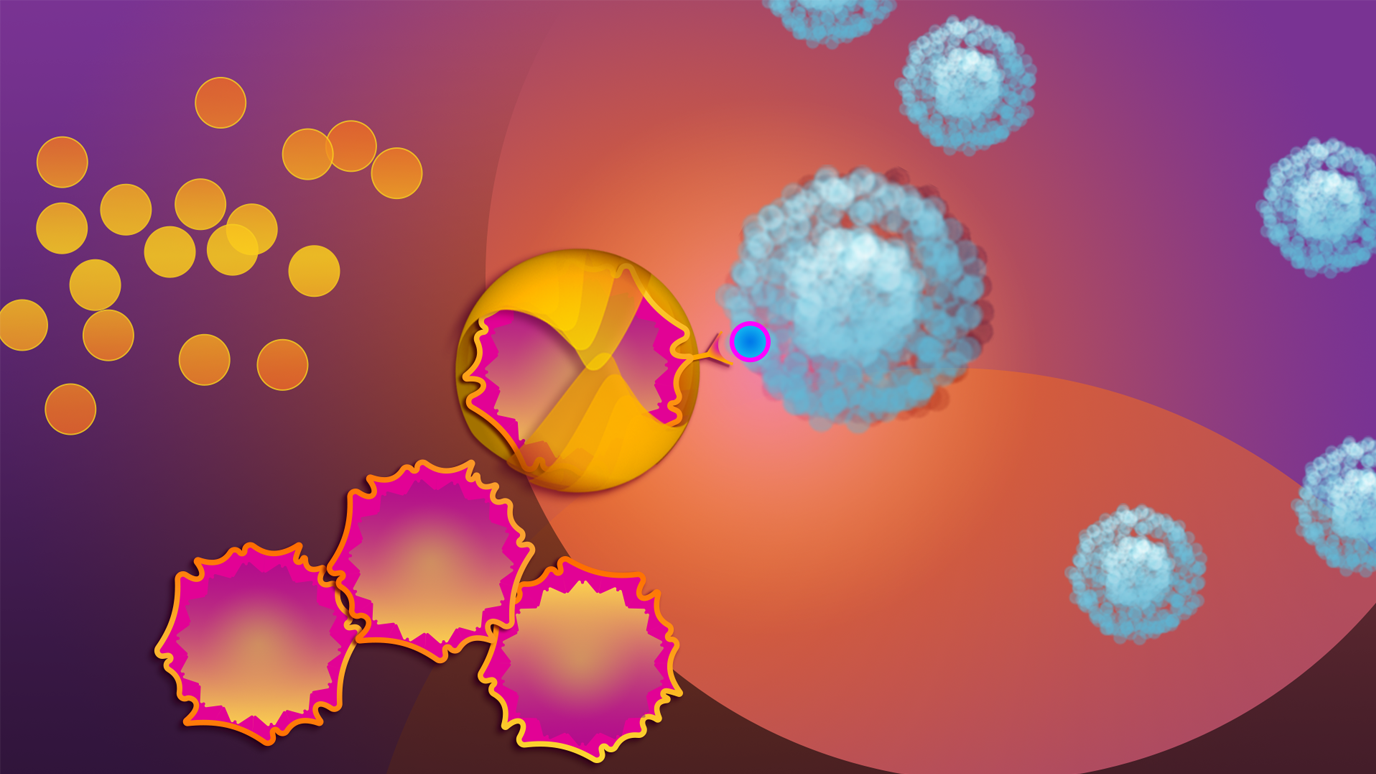
CAR T cell therapy is a type of immunotherapy that modifies T cells, so they can detect and fight cancer cells. This therapy is increasingly used to treat pediatric patients with B-cell acute lymphoblastic leukemia and, in rare cases, certain types of lymphoma.
But how is CAR T cell therapy for pediatric patients different from the treatment used for adult patients? And what are the side effects?
We spoke with registered nurse and lead pediatric stem cell transplant coordinator Sarah Featherston for answers to these questions and more. Here’s what she wants parents and caregivers to know.
How do you determine if a pediatric patient is a good candidate for CAR T cell therapy?
Currently, we have FDA-approved CAR T cells to treat relapsed and refractory patients up to age 25. These are patients whose cancer has come back or whose cancer has stopped responding to treatment.
They need to have a good performance status and the right expression, such as CD19, on the surface of cancer cells. CD19 is a marker of B cells and the target of CAR T cell therapy.
We often use CAR T cell therapy to get leukemia patients into remission, so that they can have a stem cell transplant.
Can you describe the process of CAR T cell therapy for pediatric patients?
The patient first gets a thorough workup that includes organ evaluations and testing to make sure they will be healthy enough to handle the collection process, chemotherapy and the CAR T infusion itself.
After that, the steps are as follows:
1. Cell collection
A pediatric surgeon will place a special catheter called an apheresis catheter in the patient. This is done while the child is under anesthesia. The same day, the patient undergoes cell collection in which T cells are extracted from the patient’s blood through the new catheter.
Aside from some minor discomfort where the new catheter is placed, pediatric patients generally tolerate the procedure very well. Since the catheter is only in for collection, it’s removed right after collection is complete, usually on the second day. Pediatric patients are put to sleep for catheter placement but not for removal, so they’re awake while they’re getting sutures removed and having pressure applied to that area. Naturally, they aren’t too happy about this, so with the help and expertise of our child life specialists, we talk patients through it and help put them at ease.
We work closely with the pediatric leukemia team for the best time to collect. In some cases, patients with leukemia may be taking steroids, and we try to avoid collecting while the patient is taking steroids.
2. Cell manufacturing
Our cell therapy lab freezes the cells before sending them to a specialized lab where they are genetically modified. Depending on the product, it takes about three weeks before they are sent back to the hospital.
3. Bridging therapy
When the T cells are being modified, patients will receive a small amount of chemotherapy to keep the leukemia at bay. This is called bridging therapy.
If we’ve treated the patient before, we know their treatment history pretty well. So, if a certain treatment wiped them out last time or caused the patient’s blood counts to drop extremely low, we may try something milder this time. We work closely with the pediatric leukemia team to keep the patient’s blood counts in a safe range while they’re receiving chemo.
4. Infusion
Once the modified cells arrive back at MD Anderson, the patient is given lympho-depleting chemotherapy. This means it will wipe out the lymphocytes to make room for the new CAR T cells and allow them to multiply. Then, the CAR T cells are infused into the patient’s blood. This process is quick, usually between 5 and 30 minutes. When this is an inpatient procedure, patients will spend about three weeks at the hospital before it’s safe for them to go home.
Adult CAR T patients can have their infusions done outpatient. Our goal is to offer infusion as an outpatient procedure in the future for pediatric patients as well.
5. Monitor for side effects
After the infusion, we monitor the patient for side effects. We perform several assessments to see how they’re functioning. One of the assessments is a neurological assessment called the Cornell Assessment of Pediatric Delirium (CAPD). It’s a series of questions, including ‘Is the patient making purposeful movements? Is the patient making eye contact? Is the patient expressing needs?’ We also do handwriting assessments, and if the patient is very young, we’ll have them draw pictures.
Once patients are discharged, they usually come in at least twice a week for follow-ups. We also do assessments then, and get the family involved. After all, they know the patient best. We continue assessments for eight weeks after infusion.
Once patients receive the CAR T cell infusion, we continue to follow them long-term. If there’s no disease relapse, they won’t necessarily see their leukemia or lymphoma doctor anymore. Patients will either proceed to bone marrow transplant, or they’ll be in long-term CAR T care, where we’ll watch their B cells and monitor their condition.
What are the side effects of CAR T cell therapy in pediatric patients? How do you manage them?
The two biggest side effects we see are cytokine release syndrome (CRS) and neurotoxicity, also known as Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). CRS is common, and it almost always occurs first. CRS can cause flu-like symptoms like high fever and fatigue. This is due to the high cytokine levels produced by the T cells multiplying. Generally, we begin seeing these side effects about three days after the infusion, but they can also occur later.
ICANS is identified by different grades, according to the severity of neurological symptoms. The symptoms can range from mild confusion, like the patient saying things they don’t mean by using unintended syllables, to more severe symptoms, like seizures. ICANS can happen along with CRS, but it’s rarely the first side effect. Patients can develop ICANS with CRS or once the CRS resolves.
We usually start by giving the patient an IL-6 inhibitor like tocilizumab to manage CRS symptoms. If the symptoms don’t go away after two or three doses, or symptoms become more severe, we will use additional therapies, like steroids.
Assessments are one of our earliest indicators of neurotoxicity. If a patient writes the same sentence twice a day, we can see a big change in handwriting if they begin to develop neurotoxicity. MD Anderson closely monitors patients, which helps mitigate high levels of CRS and ICANS. We can treat side effects early without destroying the CAR T cells that we want circulating.
CAR T cell therapy targets the patient’s B cells – both cancerous and non-cancerous. This can cause an extremely low count of B cells, a condition known as B-cell aplasia. This is a good indicator that a patient still has CAR T cells circulating. Patients will continue to have their B cells monitored after infusion. We’ve had some patients who have lost their B cells 30 days after infusion; for others, it happens a few months out of treatment. B-cell aplasia is not a bad thing from our perspective. If a patient has it long-term, it’s almost like they’ve got little fighters against cancer continuously monitoring their body. When they start to recover their B cells, we worry that they’re losing their CAR T cells. Luckily, we can live without B cells. B-cell aplasia is not ideal, but it’s better than cancer.
What new research is being done to advance treatment of pediatric patients with CAR T cell therapy?
CAR T cell therapy is still a new treatment, which is available for relapsed and refractory patients. Clinical trials are being conducted to see if it’s beneficial to use them for upfront therapy so that pediatric patients can avoid intense chemotherapy.
Because we’re learning new things about CAR T cell therapy, it’s a viable treatment option for more patients and families. If your child needs it, they’ll be in good hands.
Request an appointment at MD Anderson online or call 1-877-632-6789.

We can treat side effects early without destroying the CAR T cells that we want circulating.
Sarah Featherston
Lead Nurse Transplant Coordinator