- Diseases
- Acoustic Neuroma (14)
- Adrenal Gland Tumor (24)
- Anal Cancer (66)
- Anemia (2)
- Appendix Cancer (16)
- Bile Duct Cancer (28)
- Bladder Cancer (68)
- Brain Metastases (28)
- Brain Tumor (228)
- Breast Cancer (716)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (154)
- Colon Cancer (164)
- Colorectal Cancer (110)
- Endocrine Tumor (4)
- Esophageal Cancer (42)
- Eye Cancer (36)
- Fallopian Tube Cancer (6)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (6)
- Kidney Cancer (124)
- Leukemia (344)
- Liver Cancer (50)
- Lung Cancer (288)
- Lymphoma (284)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (98)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (4)
- Neuroendocrine Tumors (16)
- Oral Cancer (100)
- Ovarian Cancer (170)
- Pancreatic Cancer (166)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (144)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (236)
- Skin Cancer (294)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (60)
- Testicular Cancer (28)
- Throat Cancer (90)
- Thymoma (6)
- Thyroid Cancer (98)
- Tonsil Cancer (30)
- Uterine Cancer (78)
- Vaginal Cancer (14)
- Vulvar Cancer (18)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (20)
- Advance Care Planning (10)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (362)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (622)
- Complementary Integrative Medicine (24)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (226)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (122)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (118)
- Molecular Diagnostics (8)
- Pain Management (64)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (888)
- Research (388)
- Second Opinion (74)
- Sexuality (16)
- Side Effects (602)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (404)
- Survivorship (322)
- Symptoms (186)
- Treatment (1770)
What is CRISPR?
BY Julie Nagy
5 minute read | Published September 20, 2022
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on September 20, 2022
There are over 6 billion letters, or nucleotides, of DNA in the genome. These contain all the information needed to create an individual organism. Certain sequences of DNA, called genes, contain instructions for making proteins that determine everything about how we look and how we function. We expect there to be some differences in those sequences that lead to differences in individual humans, but sometimes these instructions have significant mutations, or changes, that can lead to serious diseases such as cancer.
Imagine having to figure out which changes in which sequences in that long string of 6 billion letters are important for targeting treatments for diseases. And, once you identify some of those important genes, how do you fix those mutations?
A game-changing discovery in 2012 of a system called CRISPR has triggered a revolution in biomedical breakthroughs over the last decade. Scientists can use it to target, edit, modify and regulate genes and put any enzyme or protein they want at any location in the genome. This allows them to find new treatment targets and understand how different genes affect cells in a way that was previously impossible.
But how do we apply CRISPR to understanding cancer? We spoke with Traver Hart, Ph.D., associate professor in Bioinformatics and Computational Biology, to learn more about CRISPR and how it could be used to advance cancer treatment.
What is CRISPR?
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. That’s a mouthful, so scientists refer to it as simply CRISPR. These are repeated sequences in the genetic code that were first found in bacteria and were later found to be part of a novel bacterial adaptive immune system against phages, which are viruses that attack bacteria.
This system combines the CRISPR DNA sequences and a set of Cas (“CRISPR associated”) proteins to identify and destroy invading viral DNA. It also embeds a sample of that viral DNA between these CRISPR sequences so that it can easily recognize and attack the same virus in the future. Thanks to this unexpected discovery in E. coli bacteria, scientists can now harness this method and use it in a similar way within human cells.
How does CRISPR work?
The main part of the CRISPR system is the Cas endonuclease, the Cas protein that cuts DNA strands. These Cas proteins can be programmed to find a 17- to 24-letter sequence by attaching a guide RNA that uniquely matches the specific DNA target. It’s similar to a key matching a lock. Researchers have a large library of guide RNAs available that can match certain parts of different genes in the human genome.
Once CRISPR is added to a cell, it searches for and binds to that matching target sequence in the DNA, and the attached Cas protein gets activated to do what scientists have asked it to do. Some Cas proteins – such as Cas9 – can cut or break the DNA. This is the original protein that was found in bacteria. Others have been engineered to turn a gene on or off without having to cut it. This allows researchers to find out more about what happens if cells make too much (upregulation) or too little (downregulation) of a certain protein and how that can affect the outcome of a cell.
How do we use CRISPR to study cancer in human cells?
For the past several decades, studies have been done in yeast cells and other model organisms where scientists can efficiently edit the genome. The discovery of CRISPR has been instrumental in changing that.
We can edit the genome directly in human cells with unprecedented ease thanks to CRISPR.
Once CRISPR cuts the target DNA, it gets repaired or replaced with a different sequence. Scientists use this method to knock out human genes in cancer cells and identify which of those genes are essential for the growth of tumor cells without harming normal cells. This allows us to nominate gene candidates for drug targets that can be very highly tumor-specific. My lab is trying to find better ways to kill tumor cells by disabling multiple genes at a time using a different Cas protein called Cas12a. This gives us more insight into how different genes and proteins work together in tumor cells to promote cancer progression.
A recent study by Yohei Yoshihama, Ph.D., and Ronald Depinho, M.D., used CRISPR to screen cancer cells and identify a protein called JMJD1C as a candidate target in castration-resistant prostate cancer. Another study by Chao Wang, Ph.D. and Junjie Chen, Ph.D., used CRISPR to screen human cancer cells growing in mouse models and discovered a protein named KIRREL, which was shown to be important for tumor suppression.
Can CRISPR fix genes in people?
While the idea of being able to fix “bad” genes to cure diseases is a worthy pursuit, science isn’t at the point to be able to safely and effectively do so – yet. Researchers are looking at how to use CRISPR to correct the genetic defects that cause beta-thalassemia and sickle cell anemia, diseases that affect the amount of hemoglobin in the body and cause patients to require constant blood transfusions. If approved, this type of therapy, called exa-cell, would become the first CRISPR-based medical treatment, which is incredibly exciting.
What’s next for CRISPR?
The possibilities are endless for the information that can be gained from using CRISPR systems and, just 10 years in, scientists have only scratched the surface. Newer Cas proteins and other enzymes are being studied, and there are still questions about how to make CRISPR more specific so that it doesn’t accidentally have unintended targets.
Here at MD Anderson, our use of CRISPR continues to lead to a better understanding of how cancer cells function and helps uncover many ways to target individual treatments specific to certain tumors that will, hopefully, one day, achieve our goal to end cancer.
Request an appointment at MD Anderson online or by calling 1-877-632-6789.
Topics
Research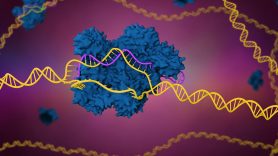
We can edit the genome directly in human cells with unprecedented ease thanks to CRISPR.
Traver Hart, Ph.D.
Researcher
