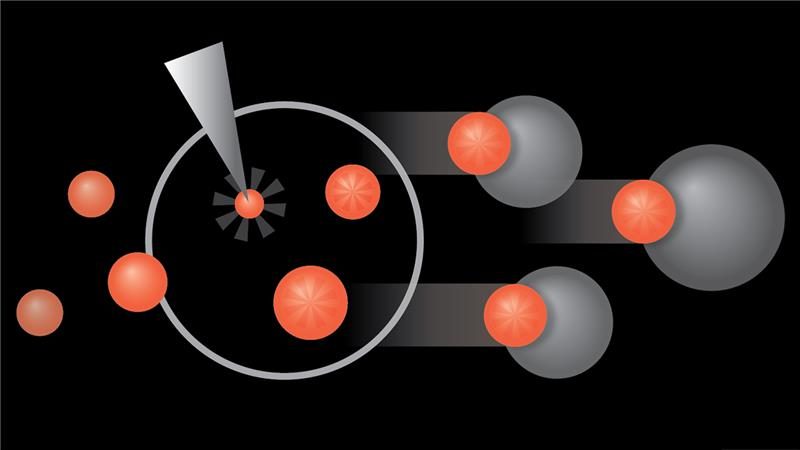
Novel CAR T cell therapy obe-cel demonstrates high response rates in adult patients with advanced B-cell ALL
Patients with relapsed or refractory CD19-positive B-cell acute lymphoblastic leukemia (ALL) who were treated with the novel anti-CD19 chimeric antigen receptor (CAR) T cell therapy, obecabtagene autoleucel (obe-cel), experienced high response rates and most did not need a subsequent stem cell transplant (SCT), according to results from the Phase Ib/II FELIX trial co-led by researchers at The University of Texas MD Anderson Cancer Center...
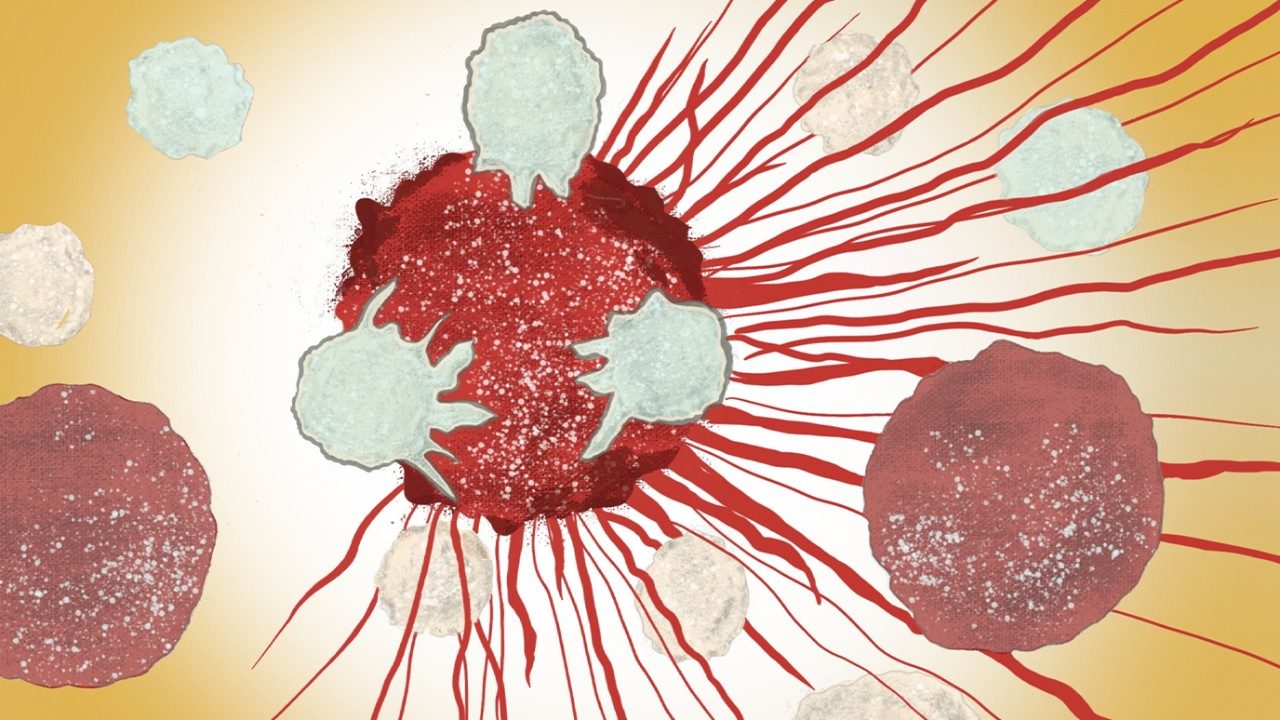
MD Anderson launches Institute for Cell Therapy Discovery & Innovation to deliver transformational new therapies
The University of Texas MD Anderson Cancer Center today announced the launch of its Institute for Cell Therapy Discovery & Innovation,...