News and Media
MD Anderson Research Highlights for...
The University of Texas MD Anderson Cancer Center’s Research Highlights provides a glimpse into recently published studies in basic, translational and clinical cancer research from MD Anderson experts. Current advances include an anti-CD19 chimeric antigen receptor (CAR) T cell therapy to treat follicular lymphoma, targeted therapies for urothelial cancers and advanced breast cancers, understanding the tumor microenvironment and immune...
Breast cancer research breakthrough
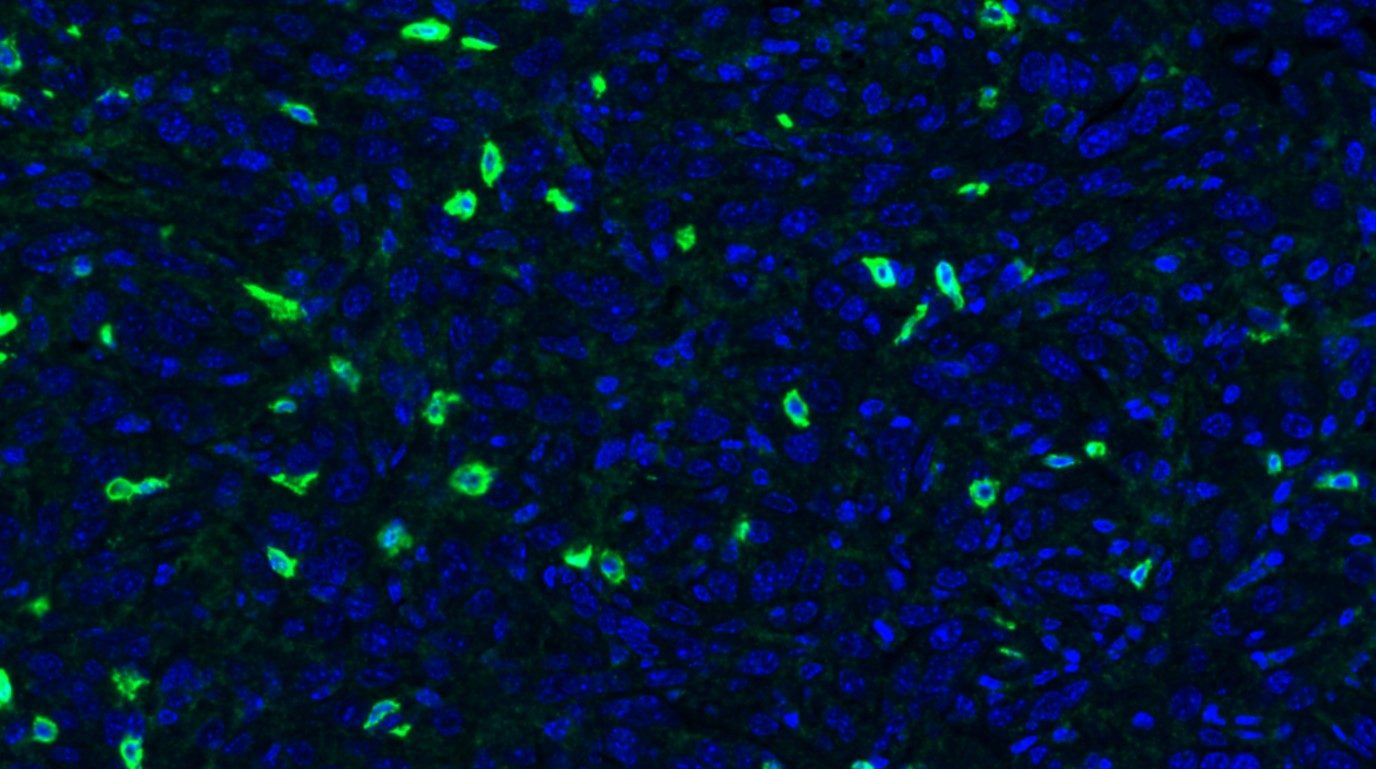
Li Ma, Ph.D., assistant professor in Experimental Radiation Oncology, reports in Nature Cell Biology that the protein ZEB1 may help breast cancer cells repair DNA damage caused by radiation treatment.
Ma’s team has demonstrated how ZEB1 helps the wily tumor cells push the panic button.
“The cancer stem cells have been shown to promote radioresistance through activation of the DNA damage response system,” says Ma. “Our studies have...
MD Anderson Research Highlights for...
The University of Texas MD Anderson Cancer Center’s Research Highlights provides a glimpse into recently published studies in basic, translational and clinical cancer research from MD Anderson experts. Clinical advances include positive data with targeted therapies for HER2 exon 20 mutant lung cancer, for older patients with mantle cell lymphoma and for BRAFV600E-mutant gliomas. Additional discoveries include insights into chromosomal...
Notable AAAS award goes to MD Anderson...
Li Ma, Ph.D., assistant professor, Experimental Radiation Oncology, is co-winner of the American Association for the Advancement of Science (AAAS) Martin and Rose Watchel Cancer Research Award.
She shares the award with Jeffrey Tyner, Ph.D., an assistant professor at Oregon Health & Science University. Ma's laboratory investigates novel determinants of tumor invasion, metastasis and therapeutic targets in breast cancer. Ma's research...
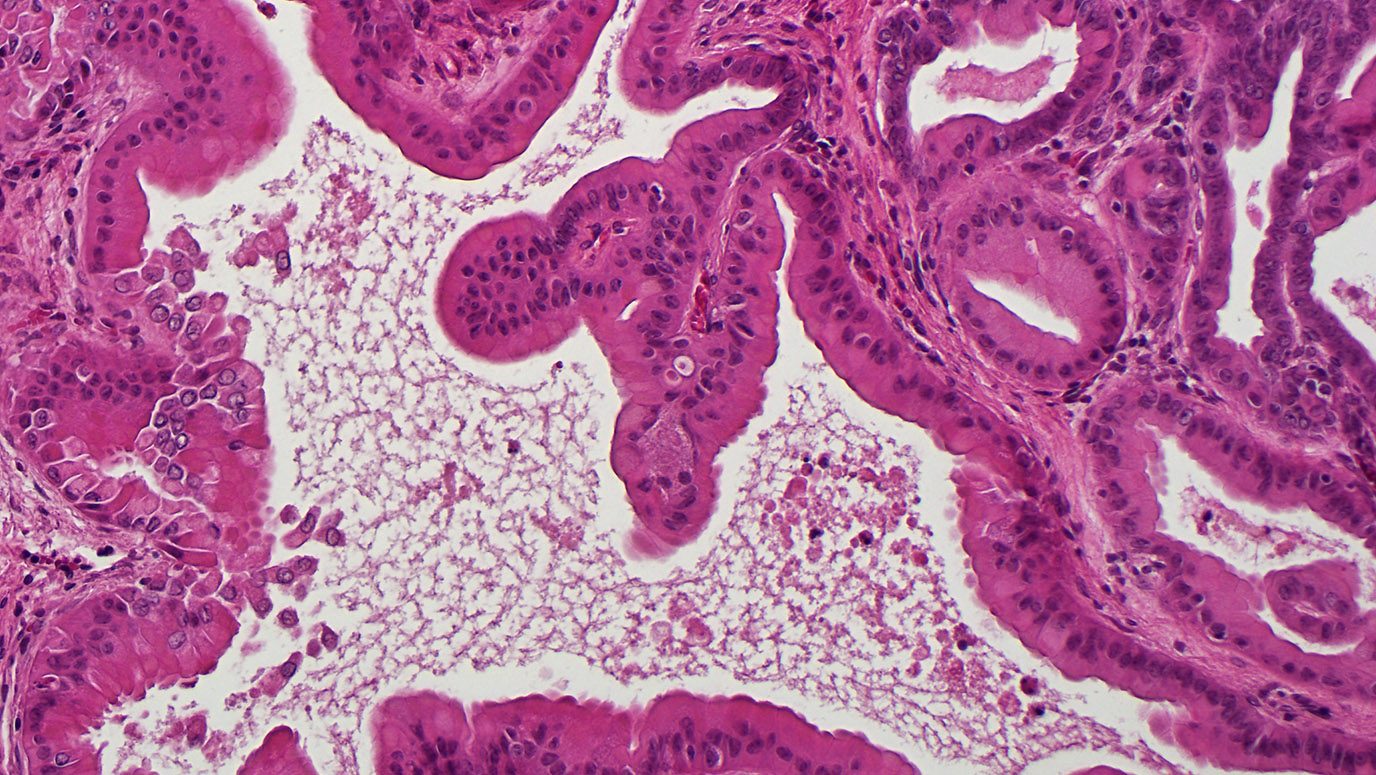
RNA thought to spread cancer shows...
Researchers at The University of Texas MD Anderson Cancer Center have discovered that a form of RNA called metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) appears to suppress breast cancer metastasis in mice, suggesting a potential new area of therapeutic investigation. The findings, published in the Oct. 22 online issue of Nature Genetics, were surprising given that MALAT1, a long non-coding RNA (lncRNA), previously...
Give Now
Your gift will help make a tremendous difference.
Research Areas
Find out about the four types of research taking place at MD Anderson.
Subscribe
Get the latest information on our cancer breakthroughs.