News
Our experts share the work of the Institute for Cell Therapy Discovery & Innovation and the advanced approaches to cancer care and research.
More about CAR NK cells
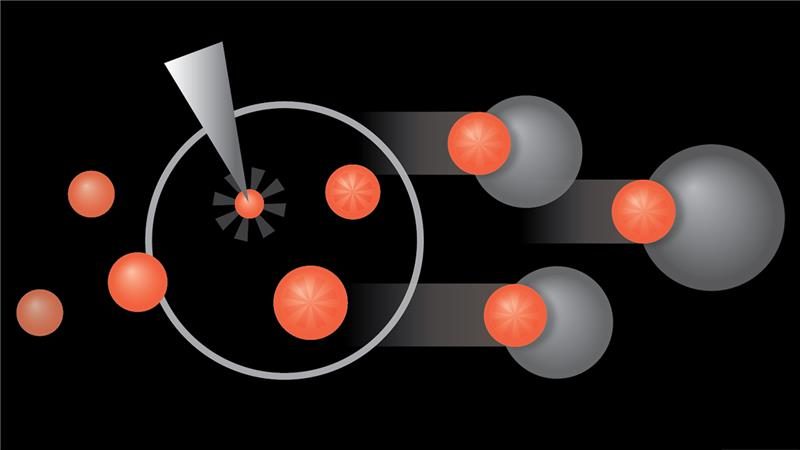
NK cells expressing interleukin-21 show promising antitumor activity in glioblastoma cells
Natural killer (NK) cells engineered to express interleukin-21 (IL-21) demonstrated sustained antitumor activity against glioblastoma stem cell-like cells (GSCs) both in vitro and in vivo, according to new research from The University of...
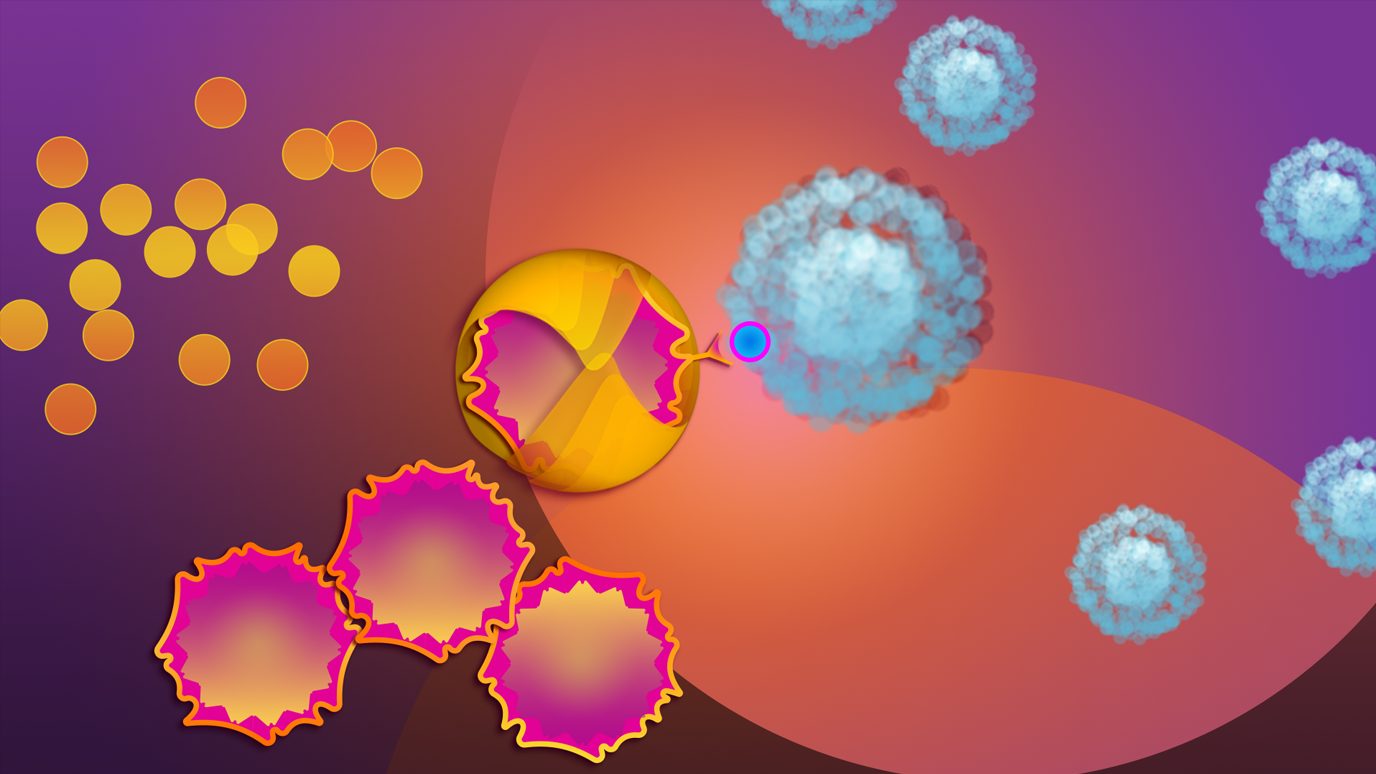
CAR NK cells with CD28 costimulation improved cell persistence and antitumor activity
Adding CD28 costimulation to cord blood-derived chimeric antigen receptor (CAR) natural killer (NK) cells targeting CD70+ cancers significantly enhanced antitumor efficacy and long-term cytotoxicity of the CAR NK cells, according to...
CD19-targeted CAR NK cell therapy achieves promising one-year results in patients with...
Researchers from The University of Texas MD Anderson Cancer Center reported promising results in a Phase I/II trial of 37 patients with relapsed or refractory B-cell malignancies who were treated with cord blood-derived chimeric antigen...
MD Anderson News
The MD Anderson Newsroom provides information about studies and findings from our cancer researchers.
Related Articles and News
MD Anderson’s Katy Rezvani, M.D., receives 2023 Honorific Award from the American Society...
Katy Rezvani, M.D., Ph.D., professor of Stem Cell Transplantation & Cellular Therapy at The University of Texas MD Anderson Cancer Center, has been honored with the E. Donnall Thomas Lecture and Prize from the American Society of...
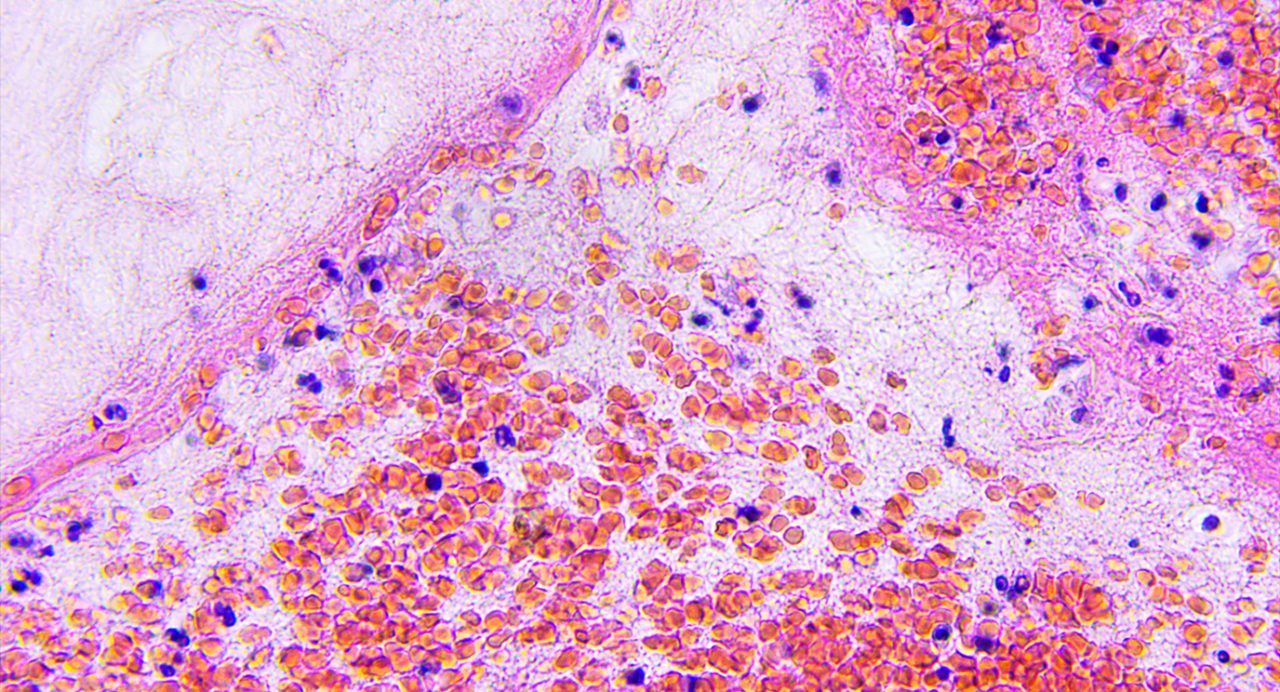
Lost metabolic fitness of CAR NK cells is key mechanism of tumor resistance
A new study led by researchers at The University of Texas MD Anderson Cancer Center discovered loss of metabolic fitness in chimeric antigen receptor (CAR) natural killer (NK) cells is a critical mechanism of resistance, with infused cells...
Give Now
Your gift will help make a tremendous difference.
Research Areas
Find out about the four types of research taking place at MD Anderson.
Subscribe
Get the latest information on our cancer breakthroughs.