Delving further into the soil

While Raghu Kalluri, M.D., Ph.D., professor and chair of Cancer Biology, started his career studying the cellular scaffolding of the kidney, his research focus has methodically shifted to examining other structural components, such as blood vessel formation. He’s currently interested in the cellular and non-cellular components of the tumor microenvironment.

Raghu Kalluri, M.D., Ph.D.
It may be surprising for those not familiar with tumor microenvironment to learn that solid tumors are composed of more non-cancerous than cancer cells. For example, pancreatic cancer adenocarcinomas are composed of up to 95% non-cancerous cells.
Clearly, cancer cells depend on the cellular environment to sustain their growth and survival, which raises the question: Are non-cancerous cells unwitting participants or enabling partners? With non-cancerous cells being the major constituents of a tumor, understanding the host microenvironmental contributions (the soil) is critical to tackling this disease.
A wound that never heals
Unresolved scarring, known as fibrosis, often occurs in the kidney, lung and liver, which leads to irreversible organ damage and eventual failure. Similarly, a tumor can be thought of as a fibrotic organ that contains cancer cells.
Valerie LeBleu, Ph.D.
Valerie LeBleu, Ph.D., assistant professor in Cancer Biology, studies how fibroblasts and mesenchymal (stromal) cells, which produce the structural components of the fibrotic matrix, replace normal tissue with scar tissue — a process known as fibrotic remodeling. While fibrosis normally occurs in a single organ responding to damage, in cancer multiple sites undergo remodeling, specifically the primary tumor site, as well as all secondary metastatic sites.
By drawing upon knowledge from a seemingly unrelated field, LeBleu is shedding light on how cancer cells co-opt stromal cells to aid in progression and metastasis. Instead of relying on assumptions about the contribution of normal cells, detailed molecular analysis now reveals how normal tissues evolve before, during and after the emergence of metastases. This will open new avenues for the rational design of the next generation of therapeutics.
Harnessing the immune system
Cancer cells can be thought to resemble virally infected cells in two ways. First, they are technically considered “self” by the immune system (as opposed to bacteria, pollen, cells from another human and other materials considered foreign), as they arise from the body’s own cells — like wolves-in-sheep’s-clothing.
Second, both the originating cell (cancer or virally infected) and healthy host cells are critical for survival. Recent research has also demonstrated that the immune response is often suppressed in the tumor microenvironment. This suppression allows cancer cells to flourish.
John Miller, Ph.D., who trained as a virologist and is now a postdoctoral fellow with Lynda Chin, M.D., professor and chair of Genomic Medicine, is working to understand how cancer cells reprogram the immune system.
Because metastatic tumors are the cause of most cancer deaths, knowing which stromal and immune components alter at fertile secondary sites will provide clues to how the immune system could be reactivated to fight cancer cells.
Miller and Chin are mining genomic data from pre- and post-treatment patient samples to identify immune components that influence disease course in melanoma. With a sense of urgency to bring this to patients, Miller hopes that his work will lead to developing immune-based therapies.
Modeling the microenvironment
Today, scientists recognize that Paget’s observations were valid and that when developing new treatments for patients, both the seed and the soil should be considered. Yet, many scientific experiments continue to rely on studying cells that are grown in plastic dishes, living conditions that are completely artificial and, therefore, inherently biased.
Taking a new path, Giulio Draetta, M.D., Ph.D., professor in Molecular and Cellular Oncology, is leveraging data from the Cancer Genome Atlas to conduct in vivo context-specific screens that will identify new genetic elements deregulated in cancer.
Once a promising target is identified, his group performs these screens in the appropriate organ context to validate the target. By directly studying patient tumor tissue samples that often resist standard of care, his research group ensures that research findings are directly applicable to patients, who currently have no therapeutic options.
In collaboration with MD Anderson’s Center for Co-Clinical Trials, they’re also generating new mouse-model systems that carefully control for lineage, genetic and microenvironmental influences. Draetta envisions that in the near future similar analyses will be available for individual patient tumors — making personalized medicine a reality.
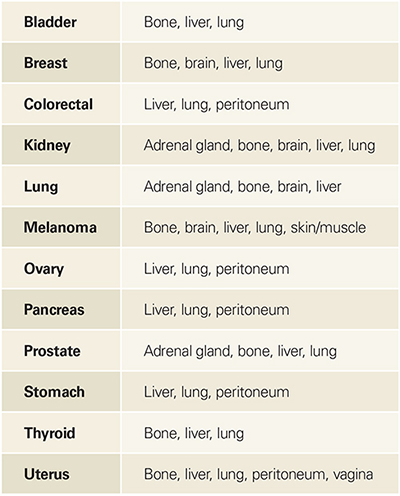
Cancer type Main sites of metastasis*
*Taken from the National Cancer Institute fact sheet on metastatic cancer.
Related story: Seed, soil and metastasis