- Diseases
- Acoustic Neuroma (14)
- Adrenal Gland Tumor (24)
- Anal Cancer (68)
- Anemia (2)
- Appendix Cancer (16)
- Bile Duct Cancer (26)
- Bladder Cancer (72)
- Brain Metastases (28)
- Brain Tumor (234)
- Breast Cancer (718)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (162)
- Colon Cancer (166)
- Colorectal Cancer (118)
- Endocrine Tumor (4)
- Esophageal Cancer (44)
- Eye Cancer (36)
- Fallopian Tube Cancer (8)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (14)
- Kidney Cancer (128)
- Leukemia (342)
- Liver Cancer (50)
- Lung Cancer (286)
- Lymphoma (278)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (100)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (6)
- Neuroendocrine Tumors (16)
- Oral Cancer (100)
- Ovarian Cancer (174)
- Pancreatic Cancer (160)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (148)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (238)
- Skin Cancer (296)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (64)
- Testicular Cancer (28)
- Throat Cancer (92)
- Thymoma (6)
- Thyroid Cancer (98)
- Tonsil Cancer (30)
- Uterine Cancer (84)
- Vaginal Cancer (18)
- Vulvar Cancer (22)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (20)
- Advance Care Planning (12)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (360)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (630)
- Complementary Integrative Medicine (22)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (232)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (126)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (116)
- Molecular Diagnostics (8)
- Pain Management (62)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (926)
- Research (390)
- Second Opinion (76)
- Sexuality (16)
- Side Effects (610)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (402)
- Survivorship (322)
- Symptoms (182)
- Treatment (1786)
Lung cancer targeted therapy hits common HER2 variants across cancers
5 minute read | Published October 02, 2019
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on October 02, 2019
Building upon the foundational discovery that the targeted therapy poziotinib is a potent and selective inhibitor of specific EGFR and HER2 mutations in lung cancer, researchers at MD Anderson have now characterized HER2 mutation type and frequency across cancer types and found that poziotinib shows activity against the most common HER2 variants seen in cancer.
The study also reports results from a phase II clinical trial, in which poziotinib resulted in an overall response rate (ORR) of 42% in 12 lung cancer patients with HER2 mutations. The findings, published online today in Cancer Cell, should guide the development of more effective treatment plans for patients with HER2 mutations across a variety of cancer types.
Despite the frequency of mutations in HER2 reported in many cancer types, there are no approved targeted therapies for tumors with HER2 mutations, explained senior author John Heymach, M.D., Ph.D., chair of Thoracic/Head and Neck Medical Oncology.
Clinical studies of different targeted therapies, known as tyrosine kinase inhibitors (TKIs), have shown mixed results across different cancer types and even varied results with different HER2 mutations in the same cancer type.
“We’ve seen studies on a number of different targeted therapies for patients with HER2 mutations, but none have proven effective, particularly for patients with mutations in exon 20,” said Heymach. “In our preclinical and clinical findings, poziotinib was the most effective targeted therapy we tested against cancers with HER2 mutations. Our findings will guide our ongoing clinical studies to bring much-needed new therapeutic options to these patients.”
Understanding the HER2 mutation landscape
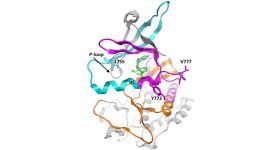
The HER2 and EGFR proteins belong to a family of signaling receptors that share similar structure and function within the cell. Mutations that stimulate the activity of EGFR are present in 10-15% of all lung cancer patients, and similar HER2 mutations are found in roughly 3% of lung cancers.
HER2 mutations have been reported in many other cancer types, but those studies report different responses to targeted therapy with no clear pattern in sensitivity, explained study lead Jacqulyne Robichaux, Ph.D., instructor of Thoracic/Head and Neck Medical Oncology.
“We hoped that understanding the mutational landscape of HER2 across cancers would allow us to determine if differential sensitivity to targeted therapy is driven by different mutational variants and if there are actionable cancer types we hadn’t previously considered for clinical trials,” says Robichaux.
To accurately catalogue the landscape of HER2 mutations, the research team analyzed 11 datasets and found mutations within 25 different cancer types. Mutations were most commonly found in bladder, bile duct and stomach cancers.
Across all cancer types, the most common mutations occurred in the tyrosine kinase signaling domain, with the majority of those found in exon 20 of the gene. Exon 20 mutations were most common in cancers of the small intestine, lung and breast. The study reports the type and frequency of specific HER2 mutations within and across cancer types.
Poziotinib is a potent inhibitor of mutant HER2
Poziotinib is a TKI that was previously abandoned due to minimal activity as a general EGFR inhibitor. Its identification as an effective targeted therapy against EGFR and HER2 with exon 20 mutations is the result of a drug repurposing pipeline created by MD Anderson’s Lung Cancer Moon Shot®, which is co-led by Heymach. The program is part of the institution’s Moon Shots Program®, a collaborative effort to accelerate the development of scientific discoveries into clinical advances that save patients’ lives.
Seeing a major unmet need in lung cancer treatment for patients with EGFR exon 20 mutations, the Moon Shot® team set out to identify targeted therapies that might be effective against these EGFR mutants. Robichaux, Heymach and others published their initial findings in a 2018 Nature Medicine study, which found that poziotinib was effective for exon 20 mutants due to its ability to fit within the altered protein structure.
In the current study, Robichaux and her team set out to systematically test TKIs against the most common HER2 mutations. The researchers screened a panel of cell lines harboring these mutations with 11 available EGFR and HER2 targeted therapies. Poziotinib was the most potent therapy tested and inhibited the most common HER2 mutations, and similar to the previous report, it was particularly effective against those mutations occurring in exon 20.
“Knowing which HER2 mutations occur across malignancies helped us to study the relationship between drug sensitivity and mutation-induced changes to the HER2 drug-binding pocket,” said Robichaux. “This understanding allows us to better match the inhibitor with the best size and shape to target HER2 mutations seen in patients.”
These cell-line results were confirmed in mouse models of HER2-mutant disease. Further, based on observations in the study, the researchers tested a combination of low-dose poziotinib with the antibody-drug conjugate therapy, trastuzumab emtansine (T-DM1). In mouse models, the combination therapy resulted in complete tumor regression in 100% of animals tested, compared to just 22% in those receiving T-DM1 alone and none of those receiving poziotinib alone.
Poziotinib effective in treating patients with HER2 exon 20 mutations
Patients with exon 20 mutations in EGFR or HER2 have traditionally had very poor responses, as other approved therapies for EGFR mutations have little to no effect in this population. Median progression-free survival (PFS) for these patients has been just two months in the past.
Based on the preclinical findings of poziotinib’s effectiveness, the investigators initiated a phase II clinical trial of poziotinib for lung cancer patients with EGFR and HER2 exon 20 mutations. Twelve patients on the trial had HER2 mutations. Of those, 50% had a partial response eight weeks after beginning treatment. Responses were confirmed two months later in five of 12 patients, representing an ORR of 42%.
Median PFS in these patients was 5.6 months, and the median duration of response was 4.6 months. The safety and toxicity profile of poziotinib was comparable to those of other FDA-approved TKIs. Most patients did have at least one dose reduction, but no patients discontinued treatment.
“These results suggest that poziotinib is well-suited to treat cancers marked by certain HER2 mutations, especially those with a constricted drug binding pocket,” said Heymach. “We look forward to future clinical studies to test the effectiveness in treating patients with HER2 mutations in a variety of cancer types, and to exploring combinations with antibody-drug conjugate therapies.”
A complete list of collaborating authors, funding support and disclosures can be found with the full paper.
MD Anderson has an institutional financial conflict of interest related to intellectual property stemming from this research and has instituted an institutional conflict of interest management and monitoring plan.