- Diseases
- Acoustic Neuroma (14)
- Adrenal Gland Tumor (24)
- Anal Cancer (66)
- Anemia (2)
- Appendix Cancer (16)
- Bile Duct Cancer (28)
- Bladder Cancer (68)
- Brain Metastases (28)
- Brain Tumor (228)
- Breast Cancer (712)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (154)
- Colon Cancer (164)
- Colorectal Cancer (110)
- Endocrine Tumor (4)
- Esophageal Cancer (42)
- Eye Cancer (36)
- Fallopian Tube Cancer (6)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (6)
- Kidney Cancer (124)
- Leukemia (344)
- Liver Cancer (50)
- Lung Cancer (288)
- Lymphoma (284)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (98)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (4)
- Neuroendocrine Tumors (16)
- Oral Cancer (98)
- Ovarian Cancer (172)
- Pancreatic Cancer (166)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (144)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (234)
- Skin Cancer (294)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (60)
- Testicular Cancer (28)
- Throat Cancer (90)
- Thymoma (6)
- Thyroid Cancer (98)
- Tonsil Cancer (30)
- Uterine Cancer (78)
- Vaginal Cancer (14)
- Vulvar Cancer (18)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (20)
- Advance Care Planning (10)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (362)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (622)
- Complementary Integrative Medicine (22)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (224)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (122)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (116)
- Molecular Diagnostics (8)
- Pain Management (64)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (882)
- Research (384)
- Second Opinion (74)
- Sexuality (16)
- Side Effects (598)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (404)
- Survivorship (324)
- Symptoms (182)
- Treatment (1764)
Immunotherapy combination is greater than the sum of its parts
4 minute read | Published November 06, 2019
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on November 06, 2019
Combining two types of immune checkpoint inhibitor fires up responses in T cells that are separate from those initiated by the two drugs individually, a research team led by Nobel Laureate Jim Allison, Ph.D., reports in the Proceedings of the National Academy of Sciences.
Administering two immunotherapy antibodies that unleash an immune attack on cancer by separately blocking the CTLA-4 and PD-1 checkpoints on T cells doesn’t have the expected additive effect of boosting the types of T cell that each drug relies on individually, Allison says.
Instead, the combination steeply reduces the presence of T cells most associated with anti-PD-1 treatment – an exhausted phenotype of cytotoxic CD8 T cells – and either converts them to or replaces them with activated effector CD8 T cells.
“There aren’t many of those exhausted T cells from anti-PD-1 therapy when you add anti-CTLA-4,” Allison says. “Their numbers are way down from PD-1 monotherapy, lower than T cells resulting from treatment with controls, and they don’t correlate with tumor response in the combination.”
“So you’re not just getting the sum of the two treatments, there’s an interaction under way here,” says Allison, chair of Immunology and co-leader of MD Anderson’s Immunotherapy platform, part of the institution’s Moon Shots Program®.
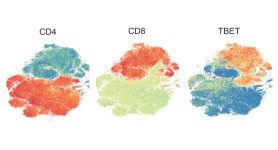
Using mass cytometry, the researchers comprehensively profiled T cell populations after treatment with anti-CTLA-4 (ipilimumab), anti-PD-1 (nivolumab) or a combination of the two drugs in a mouse model of melanoma.
Combination decreases exhausted T cells, increases activated effector T cells
The effects of anti-CTLA-4 and anti-PD-1 as separate therapies were consistent with the researchers’ earlier findings reported in Cell.
Anti-CTLA-4 treatment increased the relative frequency of T helper type 1 (Th1) CD4 effector T cells positive for PD-1, ICOS and TBET. These CD4 cells are crucial to anti-tumor response by anti-CTLA-4 therapy and are, in fact, created by anti-CTLA-4 treatment, as the team recently reported.
Also consistent with earlier findings, anti-PD-1 monotherapy increased the frequency of cytotoxic CD8 T cells with an exhausted phenotype as noted by high levels of three checkpoints on the cells: PD-1, Lag3 and Tim3.
The combination of the two drugs:
- Increased the frequency of activated CD8 effector cells.
- Elevated the presence of the CD4 effector cells beyond the levels achieved by anti-CTLA-4 alone.
- Reduced levels of exhausted CD8 cells.
All three therapeutic approaches reduced the frequency of regulatory T cells (Tregs), which dampen immune response.
The metaclusters of the activated CD8 effectors and CD4 effectors were associated with tumor reduction in the mice. The metacluster of exhausted CD8 cells was associated with tumor reduction under anti-PD-1 monotherapy but not in the combination.
“Exhausted T cells can still contribute positively to the anti-tumor response,” says Spencer Wei, Ph.D., former post-doctoral fellow in Allison’s lab and now a senior scientist at Spotlight Therapeutics. “You can think of them as tired cells, producing less than their maximal output, but still working. This is important for understanding how anti-PD-1 works. Replacing exhausted cells with fresh activated effector T cells can enhance the overall immune response.”
Possible fates of exhausted CD8 T cells
The cells are considered exhausted based on past research that connects that state to having high levels of the three checkpoints. The cells’ function was not examined in this research.
“This is what it boils down to: the exhausted phenotype CD8 cells largely go away under combination therapy,” Allison says. “So, why is that?”
It could be that the combination converts the exhausted CD8 T cells into activated effectors, although there are known barriers to that occurring. The combination’s blocking of CTLA-4 might prolong function by permitting repeated co-stimulation of the T cell via its CD28 protein, which is thwarted by the presence of CTLA-4.
Another possible explanation is that exhausted cells are simply replaced by activated CD8 effectors.
Allison’s lab has been exploring the mechanisms of immune checkpoint inhibitors in both preclinical research and in clinical samples to improve combination therapy.
“Our two main types of checkpoint blockade therapy are approved now for at least 17 types of cancer, providing durable responses for significant numbers of patients but also failing to help many others,” Allison says. “We think rationally combining these drugs with each other and with other types of therapy by understanding in detail how they work together is the best path forward to extend the benefits of immunotherapy to more patients.”
Mechanisms of action, pharmacodynamics and biomarkers can’t be inferred from the monotherapies.
“These findings mean that we cannot assume we know how to rationally put combinations together if we only know how the individual therapies work,” Wei says. “This means there's a lot more work to do to figure out how to make the combinations better.”
Larger and prospective clinical studies, as well as broader preclinical investigation, are needed to further investigate combination possibilities. Mechanistic differences may make combination therapy work better in the less immunogenic tumor types that have resisted the monotherapies.
Allison invented the field of immune checkpoint blockade by designing an antibody to block the cytotoxic T lymphocyte antigen-4 (CTLA-4) on T cells. His approach – to treat cancer by treating the immune system, not the tumor directly – led to development of the drug ipilimumab (Yervoy). His pioneering efforts were recognized with the 2018 Nobel Prize for Physiology or Medicine.
This research was supported by a grant from the Cancer Prevention and Research Institute of Texas, MD Anderson’s Odyssey Fellowship Program, the Parker Institute for Cancer Immunotherapy, the National Health and Medical Research Council of Australia and MD Anderson’s cancer center support grant from the National Cancer Institute (P30CA16672).