- Diseases
- Acoustic Neuroma (14)
- Adrenal Gland Tumor (24)
- Anal Cancer (68)
- Anemia (2)
- Appendix Cancer (16)
- Bile Duct Cancer (26)
- Bladder Cancer (72)
- Brain Metastases (28)
- Brain Tumor (232)
- Breast Cancer (714)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (2)
- Cancer of Unknown Primary (4)
- Carcinoid Tumor (8)
- Cervical Cancer (158)
- Colon Cancer (166)
- Colorectal Cancer (118)
- Endocrine Tumor (4)
- Esophageal Cancer (44)
- Eye Cancer (36)
- Fallopian Tube Cancer (8)
- Germ Cell Tumor (4)
- Gestational Trophoblastic Disease (2)
- Head and Neck Cancer (12)
- Kidney Cancer (128)
- Leukemia (342)
- Liver Cancer (50)
- Lung Cancer (286)
- Lymphoma (278)
- Mesothelioma (14)
- Metastasis (30)
- Multiple Myeloma (100)
- Myelodysplastic Syndrome (60)
- Myeloproliferative Neoplasm (6)
- Neuroendocrine Tumors (16)
- Oral Cancer (100)
- Ovarian Cancer (172)
- Pancreatic Cancer (160)
- Parathyroid Disease (2)
- Penile Cancer (14)
- Pituitary Tumor (6)
- Prostate Cancer (146)
- Rectal Cancer (58)
- Renal Medullary Carcinoma (6)
- Salivary Gland Cancer (14)
- Sarcoma (238)
- Skin Cancer (296)
- Skull Base Tumors (56)
- Spinal Tumor (12)
- Stomach Cancer (64)
- Testicular Cancer (28)
- Throat Cancer (92)
- Thymoma (6)
- Thyroid Cancer (98)
- Tonsil Cancer (30)
- Uterine Cancer (80)
- Vaginal Cancer (16)
- Vulvar Cancer (20)
- Cancer Topic
- Adolescent and Young Adult Cancer Issues (20)
- Advance Care Planning (10)
- Biostatistics (2)
- Blood Donation (18)
- Bone Health (8)
- COVID-19 (362)
- Cancer Recurrence (120)
- Childhood Cancer Issues (120)
- Clinical Trials (632)
- Complementary Integrative Medicine (22)
- Cytogenetics (2)
- DNA Methylation (4)
- Diagnosis (232)
- Epigenetics (6)
- Fertility (62)
- Follow-up Guidelines (2)
- Health Disparities (14)
- Hereditary Cancer Syndromes (126)
- Immunology (18)
- Li-Fraumeni Syndrome (8)
- Mental Health (116)
- Molecular Diagnostics (8)
- Pain Management (62)
- Palliative Care (8)
- Pathology (10)
- Physical Therapy (18)
- Pregnancy (18)
- Prevention (918)
- Research (392)
- Second Opinion (74)
- Sexuality (16)
- Side Effects (604)
- Sleep Disorders (10)
- Stem Cell Transplantation Cellular Therapy (216)
- Support (402)
- Survivorship (322)
- Symptoms (182)
- Treatment (1786)
Brain metastases recruit suppressive immune cells via novel pathway to facilitate their growth
3 minute read | Published June 15, 2020
Medically Reviewed | Last reviewed by an MD Anderson Cancer Center medical professional on June 15, 2020
MD Anderson researchers have discovered that metastatic cancer cells in the brain upregulate expression of the EZH2 gene to activate a previously unknown pathway and recruit immune-suppressive neutrophils to the brain, allowing metastatic cells to thrive. The study, published in Science Translational Medicine, suggests that targeting this neutrophil recruitment may be a useful strategy for treating brain metastases.
“To gain insights on possible strategies to treat brain metastases, we focused on finding drivers of brain metastasis that regulate the brain immune microenvironment,” says lead author Lin Zhang, Ph.D., postdoctoral fellow in Molecular and Cellular Oncology. “Based on our findings, we present an effective strategy to target immunosuppressive neutrophils to treat brain metastases using available therapies that could be fast-tracked into clinical studies.”
Identifying genetic drivers promoting brain metastasis
To discover potential therapeutic targets in brain metastases, the researchers sought to uncover genes with a role in promoting the growth and survival of brain metastatic cells. Comparing gene expression in brain metastases relative to primary cancer cells in both mouse models and matched patient samples, they identified EZH2 as a highly enriched gene in brain metastases across all datasets.
EZH2 (enhancer of zeste homolog 2) is a regulator of epigenetic modifications to DNA, which are reversible chemical changes made to control the expression of other genes. Typically, EZH2 acts to block gene expression, explains Zhang.
Using a mouse model, the researchers next confirmed that EZH2 loss impeded the development of brain metastases. Mice with cancer cells lacking EZH2 had significantly longer survival, as well as fewer and smaller brain metastases than control mice.
Interestingly, however, blocking the normal epigenetic function of EZH2 did not affect cancer cells’ ability to metastasize, indicating a novel function for this protein.
Clarifying an unexpected role for EZH2 in metastatic cells
When investigating the alternative role of EZH2, the researchers discovered that it interacts with Src, another protein enriched in brain metastases. They discovered Src works through a previously unknown mechanism to activate EZH2 and transform its role into a direct stimulator of gene expression.
Among the genes turned on by EZH2 was the oncogene c-JUN, which in turns leads to activation of a variety of pathways. In this study, increased c-JUN expression led to an increase in inflammatory cytokines, including granulocyte colony-stimulating factor (G-CSF), which played an important role in regulating the surrounding immune environment.
“G-CSF secreted from brain metastatic cells recruits neutrophils from peripheral blood into the brain, and blocking G-CSF inhibited brain metastases in mice,” says senior author Dihua Yu, M.D., Ph.D., chair ad interim of Molecular and Cellular Oncology. “We observed that neutrophils infiltrated into brain metastases had increased immunosuppressive proteins Arg1 and PD-L1 to suppress T-cell function, further promoting metastatic outgrowth.”
A new therapeutic target for brain metastases
In clinical samples from patients with breast cancer, activated Src and EZH2 were both highly enriched in brain metastases compared with matched primary tumors, and EZH2 activation was significantly correlated with neutrophil infiltration in the brain metastases.
Further, expression of a neutrophil-specific gene in brain metastases was negatively correlated with survival in an additional patient cohort. These findings suggested this pathway is of clinical importance, explains Yu.
Thus, the researchers used a mouse model of brain metastasis to study the effects of blocking Src activity with the small-molecule inhibitor, saracatinib. Treating mice with saracatinib alone reduced the levels of immunosuppressive neutrophils and increased levels of killing T cells.
When combined with immune checkpoint inhibitors, saracatinib dramatically reduced neutrophil levels, stimulated death of metastatic cells and prolonged survival in the mice.
These findings must be verified in clinical trials, but this study provides a nice proof of concept for targeting this pathway in patients with brain metastases, says Yu.
Moving forward, the researchers plan to test additional avenues to block immunosuppressive cells or activate T cells for treating brain metastases. Additionally, they will explore other targets of activated EZH2 to understand their potential role in regulating the immune landscape of brain metastases.
Research support and a full list of authors with their disclosures can be found with the full paper here.
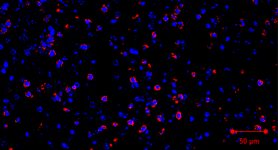
Neutrophils infiltrated into brain metastases had increased immunosuppressive proteins Arg1 and PD-L1 to suppress T-cell function, further promoting metastatic outgrowth.
Dihua Yu, M.D., Ph.D.
Physician & Researcher
