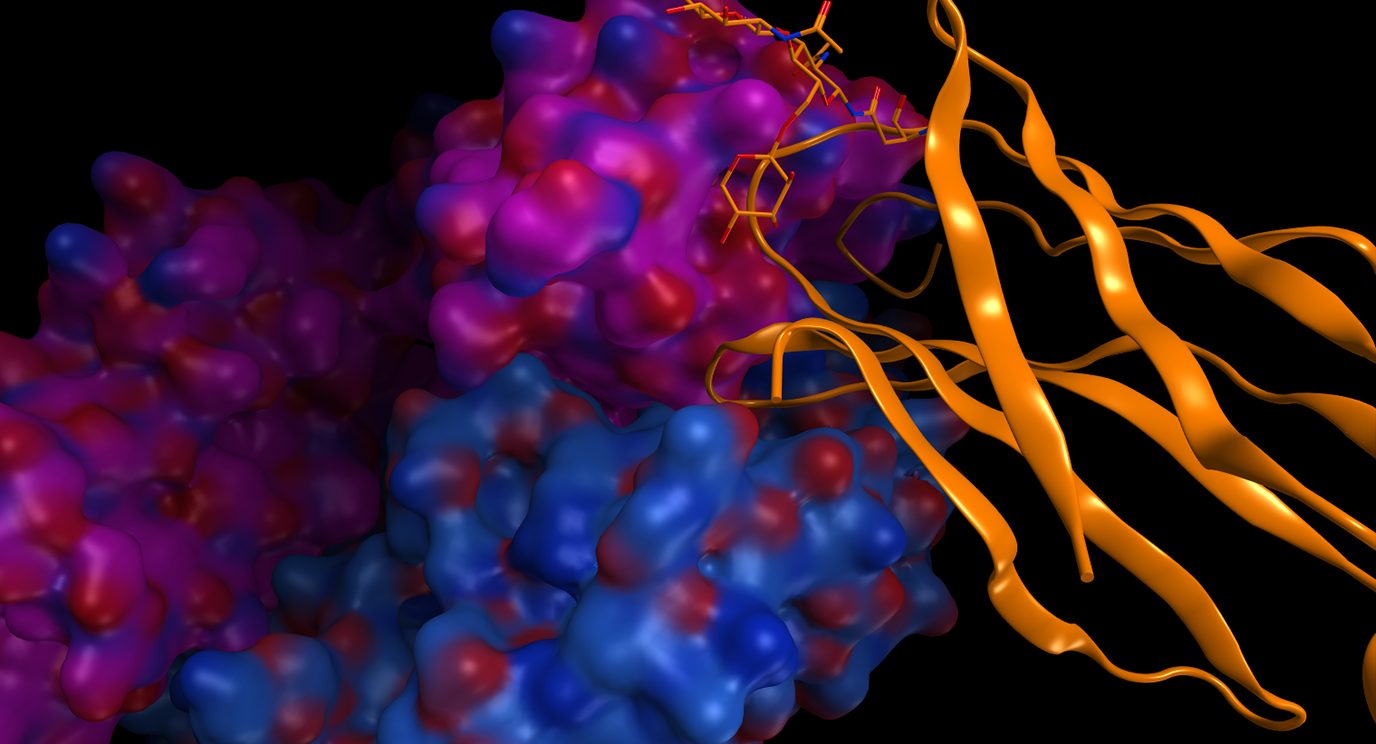
Breast, ovarian cancer trials find targeted therapies provide...
Results from two The University of Texas MD Anderson Cancer Center-led clinical trials indicate that targeted therapies led to...
Studies show early success for pre-surgical immunotherapy...
Early results from two MD Anderson studies show promising responses to neoadjuvant (pre-surgical) checkpoint inhibitors in patients...
Study identifies novel therapeutic approach for head and...
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer globally. Even with multimodality treatment, including...
AML drug may be good for KRAS-driven pancreatic cancer,...
Repurposing FDA-approved therapies is a cost-effective way to bring new treatments to patients in need, but identifying those drugs...

Predicting head and neck cancer treatment toxicities with...
MD Anderson researchers have developed the first machine learning algorithm to predict acute toxicities in patients receiving...
Moon Shot concentrates on treating, preventing high-risk...
Multiple myeloma is the second most common hematological malignancy in the United States, and it’s increasing in incidence. An...
Research finds evidence HPV vaccine is improving herd...
A study from researchers at The University of Texas MD Anderson Cancer Center found that the prevalence of the types of oral human...
Vaguely seen on images, lung nodules’ genomics outline...
Small precancerous growths in the lungs are capable of progressing to invasive lung cancer, but so little has been known about them...